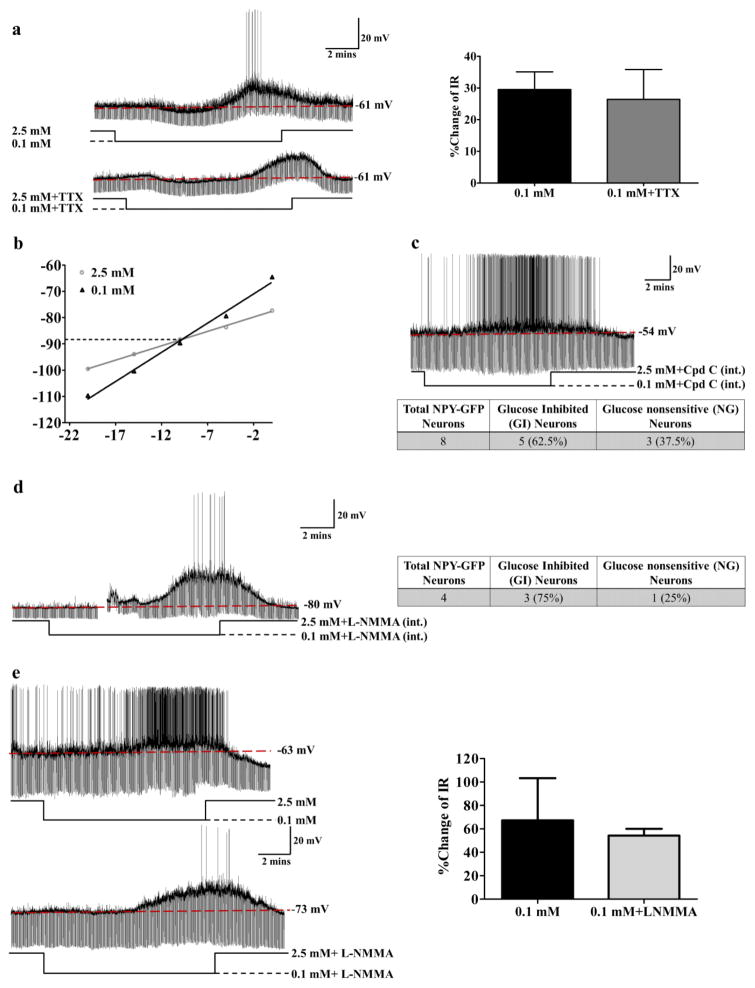
Figure 6. NPY-GI neurons use a distinct glucose sensing mechanism.
(a) There was no significant difference in the increased IR in response to decreased glucose from 2.5 to 0.1 mM in the presence or absence of TTX suggesting that low glucose directly activates NPY-GI neurons. The traces are representative whole cell current clamp recordings from an NPY-GI neuron in a brain slice. TTX blocks action potentials as shown in lower trace. The bar graphs represent %change of IR compared to that in 2.5 mM glucose (ns: no significant difference, determined by paired student’s t-test; n=6 neurons from 6 different mice evaluated for the effect of 0.1 mM glucose in the presence and absence of TTX). The data are presented as mean ± standard error of the mean (SEM). (b) Hyperpolarizing pulses from −5 to −50 pA in −5 pA steps were injected in 2.5 mM glucose and at the end of each treatment with 0.1 mM glucose. The membrane voltage measured at each pulse was used to plot the voltage-current relationship. A representative voltage-current relationship is shown. The reversal potential for this NPY-GI neurons is around −89 mV which is close to the potassium equilibrium potential (EK=−99.27) in our solutions. (c) Dialysis of NPY-GFP neurons with Compound C (Cpd C) in the patch pipette solution did not block the response of NPY neurons to decreased glucose. The trace represents the response of an NPY-GI neuron to decreased glucose after intracellular dialysis with the AMPK inhibitor Compound C. Approximately 60% (5 out of 8) of NPY neurons were recorded to be GI neurons (2 mice) after dialysis with intracellular Compound C (int.). This is identical to the expected percentage of NPY-GI neurons in the untreated population (d) Dialysis of an NPY/AgRP-GFP neuron with L-NMMA in pipette solution did not abolish the response of the NPY/AgRP neuron to decreased glucose from 2.5 mM to 0.1 mM. As shown in the table, 3 of 4 NPY/AgRP-GFP neurons (75%; 3 mice) were activated by low glucose after dialysis with intracellular L-NMMA (int.). This is similar to the expected percentage of NPY-GI neurons in the untreated population. The gap in the trace is a mechanical artifact. (e) Bath application of L-NMMA (0.1 mM) did not block the response of this NPY/AgRP-GI neuron to decreased glucose. The bar graphs represent %change of IR compared to that in 2.5 mM glucose. There is no significant difference in the % change IR in response to low glucose in the presence and absence of L-NMMA (n=3 neurons from 3 mice).