Abstract
Purpose
Patients treated with chemoradiation for head and neck cancer frequently develop dysphagia. Tissue damage to the oral tongue causing weakness and decreases in saliva production may contribute to dysphagia. Yet, effects of these variables on swallowing-related measures are unclear. The purpose of this study was (1) to determine effects of chemoradiation on tongue pressures, as a surrogate for strength, and salivary flow rates and (2) to elucidate relationships among tongue pressures, saliva production, and swallowing efficiency by bolus type.
Methods and Materials
21 patients with head and neck cancer treated with chemoradiation were assessed before and after treatment and matched with 21 healthy control participants who did not receive chemoradiation. Each participant was given a questionnaire to rate dysphagia symptoms. Videofluoroscopic evaluation of swallowing was used to determine swallowing efficiency; the Saxon test measured salivary flow rate; and the Iowa Oral Performance Instrument (IOPI) was used for oral tongue maximum and endurance measures.
Results
Results revealed significantly lower tongue endurance measures for patients post-treatment as compared to controls (p=.012). Salivary flow rates also were lower compared to pre-treatment (p=.000) and controls (p=.000). Simple linear regression analyses showed that change in salivary flow rate was predictive of change in swallow efficiency measures from pre- to post-treatment for 1mL thin liquid (p=.017), 3mL nectar-thick liquid (p=.026), and 3mL standard barium pudding (p=.011) boluses.
Conclusions
Based on these findings, it appears that chemoradiation treatment affects tongue endurance and salivary flow rate and these changes may impact swallow efficiency. These factors should be considered when planning treatment for dysphagia.
Introduction
Patients with head and neck cancer who receive chemoradiation treatment frequently develop dysphagia, or swallowing dysfunction, as a result of radiation-induced fibrosis to the critical tissues of the head and neck region [1–3]. The oral tongue is a critical structure involved in the coordination of a successful swallow and often is included in the radiation field during head and neck cancer treatment. Lingual necrosis and decreased lingual strength may result from radiotherapy to the oral tongue, base of tongue, or oropharynx [4,5]. If oral tongue function is impaired, patients will have difficulties with oral control and bolus transport, increasing the likelihood for aspiration [6–8]. Decreased maximum isometric lingual pressures, or pressures produced when the oral tongue is pushed as hard as possible against the hard palate, have been documented in patients with dysphagia [9–13]. Measures of tongue endurance, or maintenance of a percentage of maximal tongue pressure, reduce as well [14].
Previous research examining oral tongue strength and endurance in patients with head and neck cancer treated with chemoradiation has been conflicting. For example, several studies have demonstrated that neither tongue strength nor endurance measures were significantly different from pre- to post-treatment [15–17]. However, one of these studies also reported lower tongue strength as compared with a healthy cohort [15]. Comparisons between physiologic measures and tongue strength also have produced mixed results. In a study by Lazarus and colleagues [15], for several bolus types, the number of swallows per bolus was found to be lower in patients with greater tongue strength [15], which contrasted with longer oral transit times found for those with greater tongue strength [15]. The authors hypothesized that these conflicting results may be due to the role of saliva as a mediating factor [15]. While salivary flow rate was not measured in this study, the authors stated that the majority of participants reported a reduction in saliva production that affected their swallowing [15]. Given the role of saliva in providing lubrication and previous findings of longer oral transit times with reduced salivary flow [18], the authors suggested that the effects of xerostomia might have overridden the effects of tongue strength in this study.
Salivary flow rate often is reduced following radiation treatment for head and neck cancer due to high dosages to the salivary glands bilaterally [19,20]. Saliva is important in providing lubrication to the bolus that adds a source of sensory input to the oral cavity and impacts swallow initiation [21]. Patients with head and neck cancer have a significant decrease in saliva production from pre- to post-treatment and an increased number of perceived swallowing problems following treatment [22,23]. In a previous publication, we reported increased perception of swallowing dysfunction as well as decreased swallow efficiencies, higher percentages of oropharyngeal residue, and more occurrences of penetration and aspiration following chemoradiation treatment in a cohort of patients with head and neck cancer [24]. However, it is unclear how chemoradiation treatment impacts tongue strength, tongue endurance and salivary flow rate and how these predict change in swallowing-related measures following treatment. This is important information as it will impact treatment planning for patients with dysphagia following chemoradiation treatment for head and neck cancer.
The aim of the present study utilizing the same cohort as our previous study [25] was two-fold: 1) to determine the chemoradiation treatment effects on the additional variables of maximum tongue strength, tongue endurance, and salivary flow rate in this same group of head and neck cancer patients, and 2) to examine the ability of these variables to predict change in swallowing-related measures by bolus type following chemoradiation treatment. We hypothesized that: 1) tongue strength, tongue endurance, and salivary flow rate would decrease following chemoradiation treatment, 2) tongue strength, tongue endurance, and salivary flow rate would be lower in the patients following chemoradiation treatment as compared to a healthy control group; 3) change in each of these variables (tongue strength, tongue endurance, and salivary flow rate) would be predictive of change in swallowing measures from pre- to post-chemoradiation treatment with the strength of these relationships greater on thicker bolus types.
Methods and Materials
Participants
There were two groups of participants: 1) 21 patients diagnosed with head and neck cancer and treated with chemoradiation treatment, and 2) 21 age and gender matched healthy control participants. Patients were recruited through referrals from the Radiation Oncology and Medical Oncology centers and the Speech and Swallowing Clinic.
Patient participants ranged in age from 36 to 80 years (mean age= 56 years). Inclusion criteria for the patient group were: 1) diagnosis of head and neck cancer, 2) age 30 to 80 years, and 3) planned total radiation dosage of at least 50 Gy with concurrent chemotherapy. Exclusion criteria were: 1) other medical problems known to cause xerostomia and/or dysphagia (e.g., neurological problems, gastroenterologic problems), 2) previous treatment for head and neck cancer (chemotherapy, radiation, surgical intervention); 3) prior swallowing treatment, and 4) prescribed medication that could affect swallowing[26] (Table 1). Healthy control participants ranged in age from 31 to 77 years (average age= 56 years). These participants were recruited from the general community through posted flyers and were eligible for participation if they: 1) matched with a patient on gender and age (+/− 5 years), 2) did not complain of or have a history of dry mouth, 3) did not have a history of dysphagia, and 4) were not taking medication that could affect swallowing.
Table 1.
Patient Characteristics
| Characteristics | Percentages | ||||
|---|---|---|---|---|---|
| Age | 18–39 years | 40–59 years | 60–79 years | 80–99 years | |
| 5% | 61% | 29% | 5% | ||
| Sex | Male | Female | |||
| 61% | 19% | ||||
| Tumor site | Oropharyngeal | Nasopharyngeal | Other | ||
| 57% | 14% | 19% | |||
| Tumor size | T0 | T1 | T2 | T3 | T4 |
| 9% | 31% | 42% | 0% | 18% | |
| Nodal involvement | Yes | No | |||
| 95% | 5% | ||||
| Metastasis | Yes | No | |||
| 0% | 100% | ||||
| Induction chemotherapy | Yes | No | |||
| 29% | 71% | ||||
| Concurrent chemoradiation | Yes | No | |||
| 100% | 0% | ||||
| Intensity-modulated radiation delivery | Yes | No | |||
| 95% | 5% | ||||
| Surgery (tonsillectomy, neck dissection, tumor debulking) | Yes | No | |||
| 38% | 62% | ||||
| Smoking history | Yes | No | |||
| 48% | 52% | ||||
| Alcohol abuse history | Yes | No | |||
| 38% | 62% | ||||
Study Procedures
The protocol was approved by the University’s Institutional Review Board. All participants provided informed consent prior to being enrolled in the study. Each patient with head and neck cancer was evaluated twice, once before and once after chemoradiation treatment. The pre-treatment assessment point took place an average of 3.5 weeks before treatment. The post-treatment assessment was between 3 months and 1 year post-treatment (mean= 218 days; SD= 216 days; Range= 78 to 809 days) for the majority, with two patients up to two years and one just past two years due to difficulty with follow-up. The control participants were only evaluated once.
Data collection procedures at each assessment point included: 1) Patient Perception of Swallow Function Questionnaire (PPSFQ), 2) Videofluoroscopic evaluation of swallowing (VFES), 3) Saxon test for measure of salivary flow rate, and 4) Tongue strength and endurance measurements. The methods for the Patient Perception of Swallow Function Questionnaire (PPSFQ) and Videofluoroscopic Evaluations of Swallowing (VFES) have been previously published [24]. In brief, the PPSFQ consisted of 12 questions regarding swallowing function and dry mouth (see Table 2 for a list of items on the questionnaire). Each item required a rating of 1 to 7 (1= no difficulty; 7= severe difficulty).
Table 2.
PPSFQ Items
| 1. Difficulty swallowing |
| 2. Dry mouth |
| 3. Food sticks in mouth |
| 4. Food sticks in throat |
| 5. Food won’t go down |
| 6. Need water to help food go down |
| 7. Choke on food/liquid |
| 8. Cough when eating and/or drinking |
| 9. Food/liquid come back up after swallow |
| 10. Heartburn |
| 11. Wake up at night coughing and/or gagging |
| 12. Change in my sense of taste |
Each participant’s swallowing was examined using videofluoroscopy. Each participant was administered two boluses of each of the following in randomized order within the lateral plane: 1 and 10 ml of thin liquid barium, 3 and 10 ml of nectar thick liquid barium, 3 ml of thin barium pudding, 3 ml of standard barium pudding; and ¼ of a Lorna Doone cookie covered with 1 ml of EZ EM barium pudding.
Details regarding the methods utilized for data reduction of videofluoroscopic recordings have been previously published [24]. In summary, two types of measures were made: 1) approximate amount (percentage) of residue as well as approximate amount (percentage) and frequency of penetration and aspiration, and 2) selected temporal measurements of structural and bolus movement.
The timing of the following events (in seconds) were taken from videofluoroscopic recordings: (a) first backward movement of the bolus (defined as the onset of oral transit) (b) head (leading edge) of the bolus reaches the point where the ramus of the mandible crosses the tongue base, which is the point by which the pharyngeal swallow should trigger, (c) beginning of laryngeal elevation (first elevation associated with the onset of the pharyngeal stage of the swallow), and (d) end of cricopharyngeal opening (the tail of the bolus leaves the cricopharyngeal region, defined as the termination of the pharyngeal swallow). From these events, the following durational measures were then calculated (also in seconds) : oral transit time (OTT; b-a)-time it takes the bolus to move through oral cavity; pharyngeal response time (PRT; d-c)-time from onset of laryngeal elevation until bolus tail passes through the cricopharyngeal sphincter; pharyngeal delay time (PDT; c-b)-time from bolus head passing the posterior edge of mandibular ramus until initial observation of laryngeal elevation; pharyngeal transit time (PTT; d-b)-time required for bolus to move through pharynx.
The Oropharyngeal Swallow Efficiency (OPSE) for each swallow also was calculated by using the following equation:
The numerator of the OPSE equation consists of estimates of oral and pharyngeal residue as well as estimates of aspiration during and after the swallow (recorded in percentages of the total bolus (100%)). The denominator consists of three of the temporal measures recorded in seconds: oral transit time (OTT), pharyngeal delay time (PDT), and pharyngeal response time (PRT). OPSE scores typically range from 100 (100% of the bolus is swallowed in 1 second) to 140 in normal participants as the bolus becomes thicker.
The Saxon test [27] was used to measure stimulated whole saliva production. For this test, participants chewed on a 4″ × 4″ gauze pad for 2 minutes. The gauze pad was weighed before and after chewing and the difference in weight (gm) represented the amount of saliva produced in 2 minutes. The test was completed twice at each time point, and the average weight was calculated. This assessment was completed in the afternoon when saliva production is at its peak [28]. For two hours prior, participants refrained from smoking, eating, using mouth wash, or drinking anything but water.
Oral tongue strength was measured using the Iowa Oral Pressure Instrument (IOPI) [29]. The IOPI consists of an air-filled plastic bulb connected to a pressure transducer. The plastic bulb is placed between the participant’s oral tongue and hard palate. As the individual applies pressure to the bulb, any pressure change is shown on a LED display in kilopascals (kPa). To ensure accurate measurement, calibration was checked once per week.
An isometric resistance task was used to determine maximum oral tongue pressure. The IOPI bulb was placed on top of the center of the tongue. The bulb placement along the central groove of the tongue blade was demonstrated for each participant to ensure as standard placement as possible [30]. Instructions were then to “push the bulb against the roof of your mouth as hard as possible using your tongue and not your teeth.” This measure was completed three times with two minutes of rest in between each trial. The greatest value for the three trials was the maximum pressure produced (Pmax).
A durational measure was used to determine oral tongue endurance. Each individual’s Pmax was set in the IOPI. 50% of the Pmax was manually calculated at entered into the device as a target for the endurance task. The participant was instructed to push the bulb against the hard palate with their tongue and to hold it as long as possible. The display showed a series of lights that changed from red to green as the participant reached 50% of the Pmax. The participant was instructed to keep the green light on as long as possible. The length of time that the participant maintained 50% of the Pmax was recorded (sec). This measure was completed 3 times with 2 minutes of rest in between. The greatest value for the 3 trials was the maximum endurance value (Emax).
Statistical Analysis
Repeated measures analyses of variance were conducted for each dependent variable (salivary flow rate, tongue maximum pressures, tongue endurance measures) to test for significant differences from pre- to post-treatment. Between-groups analyses of variance also were conducted for each dependent variable to test for differences between groups (control versus patients pre- and post-treatment). Pearson product-moment correlation coefficients allowed for examination of associations among the dependent variables at the post-treatment point. These same correlations were calculated using change scores (difference between post- and pre-treatment points) for saliva weight, tongue maximum pressures, tongue endurance measures, and biomechanical measures (OTT, PDT, PRT, PTT, OPSE) for each bolus type. Simple linear regression analyses with change scores were used to determine whether change in saliva weight, tongue maximum pressures, and tongue endurance measures following treatment were predictive of change in overall OPSE values as well as OPSE by bolus type. The critical value for obtaining statistical significance was set at α = 0.05. If a participant had missing data due to difficulties with equipment or the image, then data missing specific to each analysis were excluded. Analyses were conducted using IBM SPSS (Version 22) [31].
Results
Treatment Effects on Tongue Pressures and Salivary Flow Rate
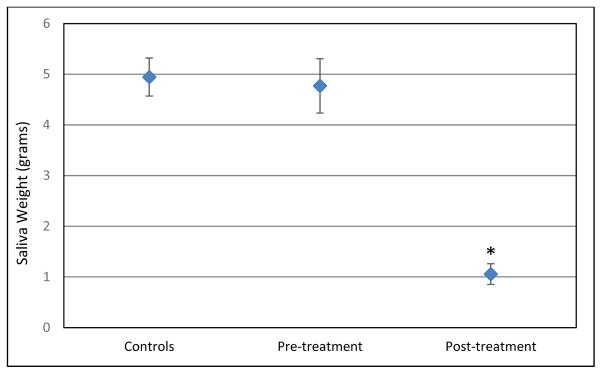
Although no significant differences in pre- to post-treatment tongue maximum pressure measurements (F(1,14)=1.08, p=.316) or endurance pressure measurements (F(1,14)=2.34, p=.148) were noted, significant decreases in salivary flow rates were observed (F (1,20)=58.26, p=.000, partial eta squared= .744) (Figure 1).
Figure 1.
Mean (average) saliva weights with standard error bars for each group. Asterisk= statistical significance. This graph shows a significant decrease in mean saliva weight from pre- to post-treatment as well as a significant difference between the post-treatment saliva weights and the saliva weights of the control group.
There was no significant difference between the patient group at pre-treatment point and the control group in Pmax values (F(1,34)=1.96, p=.171), E max values (F(1,34)=2.17, p=.15), or salivary flow rate (F(1,40)= .07, p=.792).
While Pmax was lower post treatment in the patient group versus the control group, this difference approached but did not reach significance (F(1,40)=3.92, p=.055). Emax (F(1,39)=6.9, p=.012; partial eta squared= .15) and salivary flow rate (F(1,40)=82.8, p=.000; partial eta squared= .67) were found to be significantly lower post-treatment in the patient group as compared to the control group. Table 3 includes the means (averages) and standard deviations for Pmax, Emax, and salivary flow rate by group and assessment point.
Table 3.
Means for Salivary Flow Rate, Tongue Maximum Pressures, and Endurance Measures
| Variables | Control Participants | Pre-treatment | Post-treatment | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Salivary flow rate (SFR) | 4.94 g | 1.72 | 4.77 g | 2.46 | 1.06 g | 0.94 |
| Tongue maximum pressures (Pmax) | 58 kPa | 15.8 | 51 kPa | 13.7 | 49 kPa | 13.4 |
| Tongue endurance measures (Emax) | 68.9 secs | 43.8 | 45 secs | 16.4 | 42 secs | 15.0 |
Correlations among Salivary Flow Rate, Tongue Pressures, PPSFQ scores, and Swallowing Biomechanics in the Patient Group at the Post-Treatment Point
There were no significant correlations among Pmax, Emax, and saliva weight values at the post-treatment point.
In the patient group at the post-treatment point, there was a moderate, negative correlation between ratings on the PPSFQ individual item “I have a dry mouth” and salivary flow rate (r=−.492, n=21, p=.024) with higher ratings associated with lower flow rates. Ratings on another PPSFQ item “I wake up at night coughing and gagging” were also moderately, negatively correlated with salivary flow rate in the patient group post-treatment (r=−.453, n=21, p=.04), with higher ratings associated with lower flow rates (Table 4).
Table 4.
Correlations between PPSFQ items and Saliva Weight
| Items on PPSFQ | Correlation | p value |
|---|---|---|
| Difficulty swallowing | −.244 | .286 |
| Dry mouth | −.492* | .024 |
| Food sticks in mouth | −.054 | .816 |
| Food sticks in throat | −.214 | .352 |
| Food won’t go down | −.223 | .331 |
| Need water | −.239 | .297 |
| Choke | −.215 | .348 |
| Cough | −.233 | .309 |
| Comes back up | −.133 | .564 |
| Heartburn | −.206 | .371 |
| Wake at night | −.453* | .039 |
| Change in taste | −.206 | .371 |
| Total scores | −.374 | .095 |
There were no significant correlations between salivary flow rate and swallowing biomechanical measures averaged across bolus type at the post-treatment point. However, a significant moderate, positive correlation was found between OPSE on the 10mL thin liquid bolus and salivary flow rate (r=.461, p=.047).
In the patient group at the post-treatment point, a negative correlation between overall amounts of oral residue and Pmax approached but did not reach significance (r= −.43, p=.055). Moderate, negative correlations were found between oral residue on 10mL thin liquid bolus and 10mL nectar-thick liquid bolus and Pmax (r=−.48, p=.036; r=−.55, p=.012, respectively).
Regression Analyses with Change Scores
Change in saliva weight from pre- to post-treatment for the patient group was not predictive of change in Pmax (F(1,13)= .134; p=.72) or Emax (F(1,13)= .002; p=.964).
While change in saliva weight from pre- to post-treatment did not significantly predict change in average OPSE scores, this relationship approached significance (F(1, 19)= 4.07, p=.058). Similarly, change in tongue endurance measures did not significantly predict change in average OPSE scores (F(1,13)= 3.88, p=.07) or OTT values (F(1,13)= 3.83; p=.07) but these relationships approached significance.
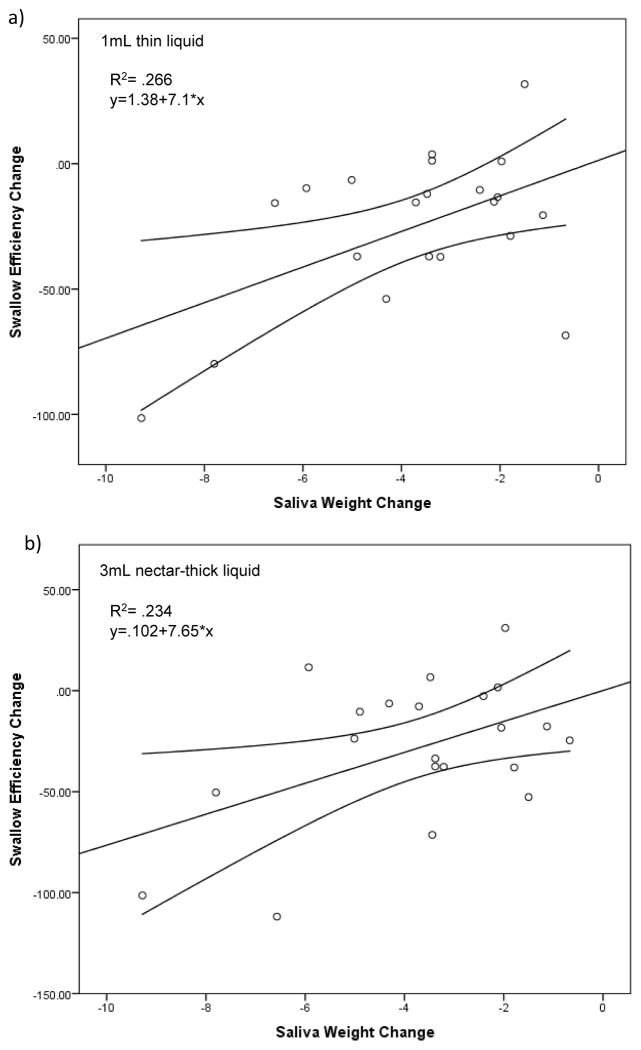
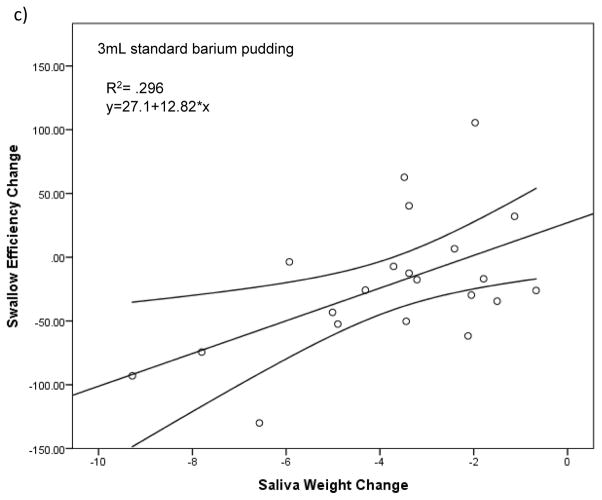
Change in saliva weight from pre- to post-treatment significantly predicted change in OPSE scores on 1mL thin liquid boluses (F(1,19)= 6.89; p=.017), 3mL nectar-thick liquid boluses (F(1,19)= 5.80; p=.026), and 3mL standard barium pudding boluses (F(1,19)= 8.01; p=.011). Change in saliva weight accounted for 6.9%, 5.8%, and 8% of the variability in OPSE scores for 1mL thin liquid, 3mL nectar-thick, and 3mL standard barium pudding boluses, respectively. The regression equation for 1mL thin liquid was: predicted change in OPSE= 1.38 + 7.09 × (change in saliva weight) (Figure 2a). The regression equation for 3mL nectar-thick liquid was: predicted change in OPSE= .102 + 7.65 × (change in saliva weight) (Figure 2b). The regression equation for 3mL standard barium pudding was: predicted change in OPSE= 27.1 + 12.82 × (change in saliva weight) (Figure 2c).
Figure 2.
Each scatterplot (a–c) represents statistically significant (p<.05) simple linear regression models with saliva weight as a predictor of swallow efficiency for each bolus type. Line of best fit and 95% confidence intervals are shown. The graph illustrates that a larger reduction in salivary flow results in a negative change in swallow efficiency for these specific bolus types.
Discussion
The purpose of this study was to examine chemoradiation treatment effects on oral tongue strength and salivary flow rate and to define relationships with perceived swallowing difficulty and swallowing biomechanics by bolus type. We hypothesized that chemoradiation treatment for patients with head and neck cancer would lead to changes in oral tongue strength and salivary flow rate. We also hypothesized that these variables would predict change in swallowing measures, with stronger relationships observed on thicker boluses. Our findings supported this hypothesis in part in that we discovered significant decreases in salivary flow rates from pre- to post-treatment and significantly lower tongue endurance measures for the patients post-treatment as compared to the control group. Additionally, salivary flow rate was found to significantly predict swallow efficiency for specific bolus types, with the strongest relationships occurring on thicker boluses.
The decrease in salivary flow rate from pre- to post-chemoradiation treatment observed in this study is consistent with previous findings [19,20,32]. It is known that radiation damage to salivary gland tissue leads to lower amounts of saliva production [19,20]. Patients post-treatment with lower salivary quantity reported greater mouth dryness [33–37]. Those with lower salivary quantity also reported greater frequency of waking in the night, consistent with previous research.[38], [39] Symptoms of mouth dryness in those with lower salivary production, especially in individuals who are mouth breathers during sleep, may lead to a need to drink water in the night more frequently. Interestingly, there are patients who have been found to report mouth dryness without a documented decrease in salivary quantity [40–44]. Given the finding of only a moderate correlation between xerostomia and hyposalivation in this study, it may be that other aspects of saliva (e.g., viscosity and alterations in protein components) are contributing to perceived mouth dryness.
While salivary flow rate is known to decrease following chemoradiation, its impact on swallowing biomechanics is not well-established [32,45]. Findings from this study suggest that a reduction in salivary flow does contribute to changes in swallow efficiency and that these effects may be dependent upon bolus type. A decrease in salivary quantity was found to significantly predict change in swallow efficiency from pre- to post-treatment for 1mL thin liquid, 3mL nectar-thick, and 3mL standard barium pudding boluses only. This relationship was strongest for the standard barium pudding boluses suggesting that, while salivary lubrication is necessary for all bolus types, the influence of inadequate saliva production on swallowing safety may be greater with thicker bolus consistencies.
There was a lack of significant change in tongue maximum pressures and tongue endurance pressure from pre- to post-treatment [16]. One possible explanation is the presence of the tumor pre-treatment could lead to a reduction in tongue strength that may be reversed with effective treatment of the tumor (tumor debulking), thereby minimizing the change observed in tongue strength from pre- to post-treatment [16]. Interestingly, while lower than the control group (68.9 secs) in this study, tongue endurance measures at each assessment point (pretreatment=45 secs; posttreatment=42 secs) were similar to those previously reported for healthy participants [15,46]. This highlights the need for more normative data on tongue endurance for comparison with dysphagic patients.
Significantly lower tongue muscle endurance measures in the patient group posttreatment compared to the control group may be a sign of tongue muscle fatigue [46,47]. Muscle endurance is the ability to maintain repeated muscle contractions against resistance for an extended period of time [48], and a reduction in muscle endurance may represent fatigue [46]. As demonstrated by Kays et al [46], tongue endurance measures have been found to decrease following a meal in both young and older healthy adults. Lower tongue muscle endurance may place patients at risk for greater fatigue following a meal, thereby increasing the risk of airway invasion and swallow inefficiency. However, it is important to note that the slight decrease in tongue endurance from pre- to post-treatment was not statistically significant (45 secs versus 42 secs), which may indicate this patient group is at increased risk of fatigue even before undergoing chemoradiation treatment.
With higher volume liquid boluses, lower tongue maximum pressures were associated with greater oral residue. Consistent with previous studies in healthy participants [49–51], tongue strength may have a greater influence on bolus clearance with larger volumes. Despite the hypothesis that salivary flow rate mediates the effect of tongue strength or endurance on swallowing [15], no clear associations among these variables were established.
There were several limitations to this study. The patients in our head and neck cancer group varied in terms of tumor site, exact chemoradiation treatment protocol, and smoking/alcohol history. The time from completion of treatment to post-treatment assessment varied between patients. Due to difficulty with follow-up closer to treatment completion secondary to acute effects, three patients were assessed beyond one year. While possible for the results to have been affected by these later follow-up points, given that the majority of patients were seen within a year from treatment completion, it seems unlikely. Also, measures of tongue strength and endurance were taken in an isolated task and not during swallowing. Relationships may become stronger with measures taken during a swallowing task. Additionally, while utilized in previous studies [22,23], the Patient Perception of Swallowing Function Questionnaire (PPSFQ) has not yet been validated and reliability has not been established.
Despite these limitations, findings of this study suggest that decreases in saliva production following chemoradiation may at least partially underlie the previously documented [25,52,53] decreases in overall efficiency of the swallow in patients with head and neck cancer treated with chemoradiation, especially with thicker bolus types. This has important implications for clinicians treating these patients in that they should consider recommending saliva substitutes, water swallows following thicker boluses, and modifications of thicker foods to improve swallow efficiency. Findings related to tongue strength and endurance only partially support our hypotheses. While there were decreases in both tongue strength and tongue endurance from pre- to post-treatment, these changes did not reach statistical significance. Additionally, neither tongue strength nor tongue endurance were found to be predictive of swallow efficiency. The post-treatment tongue endurance values were significantly lower than the control group which may have clinical significance in terms of fatigue during meals but larger studies are required to address this. Overall, results of this study support consideration of salivary flow rate and tongue pressures when designing treatment plans and in the management of swallowing dysfunction following chemoradiation treatment.
Acknowledgments
The authors would like to thank Jeri Logemann, PhD, CCC-SLP for her guidance and mentorship during completion of this study. Additionally, thanks to Muveddet Harris for assistance with data reduction as well as Kristin Larsen, Sharon Veis, Cory Atkinson, and Megan Schliep for assistance with participant recruitment. This work was performed at Northwestern University, Department of Communication Sciences and Disorders in Evanston, IL as well as Northwestern Memorial Hospital in Chicago, IL. The manuscript was partially prepared at the William S. Middleton Veteran Affairs Hospital in Madison, WI; GRECC manuscript #2016-012. The views and content expressed in this article are solely the responsibility of the authors and do not necessarily reflect the position, policy, or official views of the Department of Veteran Affairs or the U.S. government.
Footnotes
Disclosures
There are no relevant conflicts of interest to report for Nicole Rogus-Pulia, Bharat Mittal, Marge Pierce, Charles Larson, Steve Zecker, Korey Kennelty, Amy Kind, or Nadine Connor.
Contributor Information
Nicole M. Rogus-Pulia, Advanced Geriatrics Fellow, Geriatric Research Education and Clinical Center (GRECC), William S. Middleton Memorial Veterans Hospital, Adjunct Professor, University of Wisconsin-Madison, Department of Medicine, School of Medicine and Public Health.
Charles Larson, Northwestern University, Department of Communication Sciences and Disorders, Frances Searle Building, Evanston, IL 60208
Bharat B Mittal, Professor and Chairman, Department of Radiation Oncology, Northwestern University, Feinberg School of Medicine, Chicago, IL.
Marge Pierce, Nursing Clinical Coordinator, Radiation Oncology, Northwestern Memorial Hospital, Chicago, IL, 60611.
Steven Zecker, Northwestern University, Department of Communication Sciences and Disorders, Frances Searle Building, Evanston, IL 60208
Korey Kennelty, Advanced Geriatrics Fellow, Geriatric Research Education and Clinical Center (GRECC), William S. Middleton Memorial Veterans Hospital, Assistant Adjunct Professor, University of Wisconsin-Madison, School of Pharmacy.
Amy Kind, Associate Professor, University of Wisconsin-Madison, Department of Medicine, School of Medicine and Public Health, Attending Physician, Geriatric Research Education and Clinical Center (GRECC), William S. Middleton Memorial Veterans Hospital
Nadine P. Connor, Professor, University of Wisconsin-Madison, Department of Surgery, School of Medicine and Public Health, Department of Communication Sciences and Disorders.
References
- 1.Manikantan K, Khode S, Sayed SI, Roe J, Nutting CM, Rhys-Evans P, Harrington KJ, Kazi R. Dysphagia in head and neck cancer. Cancer Treatment Reviews. 2009 Dec;35(8):724–732. doi: 10.1016/j.ctrv.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen NP, Frank C, Moltz CC, Karlsson U, Nguyen PD, Ward HW, Vos P, Smith HJ, Huang S, Nguyen LM, Lemanski C, Ludin A, Sallah S. Analysis of factors influencing Dysphagia severity following treatment of head and neck cancer. Anticancer Res. 2009 Aug;29(8):3299–3304. [PubMed] [Google Scholar]
- 3.Mittal BB, Pauloski BR, Haraf DJ, Pelzer HJ, Argiris A, Vokes EE, Rademaker A, Logemann JA. Swallowing dysfunction--preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys. 2003 Dec;57(5):1219–1230. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus CL, Logemann JA, Kahrilas PJ, Mittal BB. Swallow recovery in an oral cancer patient following surgery, radiotherapy, and hyperthermia. Head Neck. 1994 Jun;16(3):259–265. doi: 10.1002/hed.2880160309. [DOI] [PubMed] [Google Scholar]
- 5.David JM, Paty E, Bachaud JM, Vignaud JR. Lingual necrosis after radiotherapy. Radiation arteritis? Rev Stomatol Chir Maxillofac. 1989;90(5):334–336. [PubMed] [Google Scholar]
- 6.Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995 May;108(5):1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- 7.Chi-Fishman G, Stone M, McCall GN. Lingual action in normal sequential swallowing. J Speech Lang Hear Res. 1998 Aug;41(4):771–785. doi: 10.1044/jslhr.4104.771. [DOI] [PubMed] [Google Scholar]
- 8.Steele CM, Cichero JAY. Physiological factors related to aspiration risk: a systematic review. Dysphagia. 2014 Jun;29(3):295–304. doi: 10.1007/s00455-014-9516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida N, Tohara H, Hara K, Kumakura A, Wakasugi Y, Nakane A, Minakuchi S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr Gerontol Int. 2016 Jan; doi: 10.1111/ggi.12715. [DOI] [PubMed] [Google Scholar]
- 10.Steele CM, Bayley MT, Peladeau-Pigeon M, Nagy A, Namasivayam AM, Stokely SL, Wolkin T. A Randomized Trial Comparing Two Tongue-Pressure Resistance Training Protocols for Post-Stroke Dysphagia. Dysphagia. 2016 Mar; doi: 10.1007/s00455-016-9699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Day C, Frank E, Montgomery A, Nichols M, McDade H. Repeated tongue and hand strength measurements in normal adults and individuals with Parkinson’s disease. Int J Orofacial Myology. 2005 Nov;31:15–25. [PubMed] [Google Scholar]
- 12.Easterling C, Antinoja J, Cashin S, Barkhaus PE. Changes in tongue pressure, pulmonary function, and salivary flow in patients with amyotrophic lateral sclerosis. Dysphagia. 2013 Jun;28(2):217–225. doi: 10.1007/s00455-012-9436-7. [DOI] [PubMed] [Google Scholar]
- 13.Steele CM, Bailey GL, Polacco REC, Hori SF, Molfenter SM, Oshalla M, Yeates EM. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int J Speech Lang Pathol. 2013 Oct;15(5):492–502. doi: 10.3109/17549507.2012.752864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000 Feb;43(1):256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res. 2000 Aug;43(4):1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus C, Logemann JA, Pauloski BR, Rademaker AW, Helenowski IB, Vonesh EF, Maccracken E, Mittal BB, Vokes EE, Haraf DJ. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007 Jul;29(7):632–637. doi: 10.1002/hed.20577. [DOI] [PubMed] [Google Scholar]
- 17.Chang CW, Chen SH, Ko JY, Lin YH. Early radiation effects on tongue function for patients with nasopharyngeal carcinoma: a preliminary study. Dysphagia. 2008 Jun;23(2):193–198. doi: 10.1007/s00455-007-9128-x. [DOI] [PubMed] [Google Scholar]
- 18.Hughes C, Baum B, Fox P, Marmary Y, Yeh CK, Sonies B. Oral-pharyngeal dysphagia: A common sequela of salivary gland dysfunction. Dysphagia. 1987;1(4):173–177. [Google Scholar]
- 19.Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992;28(2–3):457–462. doi: 10.1016/s0959-8049(05)80076-0. [DOI] [PubMed] [Google Scholar]
- 20.Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 2006 Dec;107(11):2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 21.Peyron MA, Gierczynski I, Hartmann C, Loret C, Dardevet D, Martin N, Woda A. Role of physical bolus properties as sensory inputs in the trigger of swallowing. PLoS ONE. 2011;6(6):e21167. doi: 10.1371/journal.pone.0021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logemann JA, Smith CH, Pauloski BR, Rademaker AW, Lazarus CL, Colangelo LA, Mittal B, MacCracken E, Gaziano J, Stachowiak L, Newman LA. Effects of xerostomia on perception and performance of swallow function. Head Neck. 2001 Apr;23(4):317–321. doi: 10.1002/hed.1037. [DOI] [PubMed] [Google Scholar]
- 23.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Mittal B, Gaziano J, Stachowiak L, MacCracken E, Newman LA. Xerostomia: 12-month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation. Head Neck. 2003 Jun;25(6):432–437. doi: 10.1002/hed.10255. [DOI] [PubMed] [Google Scholar]
- 24.Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in Swallowing Physiology and Patient Perception of Swallowing Function Following Chemoradiation for Head and Neck Cancer. Dysphagia. 2014 Jan; doi: 10.1007/s00455-013-9500-y. [DOI] [PubMed] [Google Scholar]
- 25.Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia. 2014 Apr;29(2):223–233. doi: 10.1007/s00455-013-9500-y. [DOI] [PubMed] [Google Scholar]
- 26.Stoschus B, Allescher HD. Drug-induced dysphagia. Dysphagia. 1993;8(2):154–159. doi: 10.1007/BF02266997. [DOI] [PubMed] [Google Scholar]
- 27.Kohler PF, Winter ME. A quantitative test for xerostomia. The Saxon test, an oral equivalent of the Schirmer test. Arthritis Rheum. 1985 Oct;28(10):1128–1132. doi: 10.1002/art.1780281008. [DOI] [PubMed] [Google Scholar]
- 28.Papagerakis S, Zheng L, Schnell S, Sartor MA, Somers E, Marder W, McAlpin B, Kim D, McHugh J, Papagerakis P. The circadian clock in oral health and diseases. J Dent Res. 2014 Jan;93(1):27–35. doi: 10.1177/0022034513505768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams V, Mathisen B, Baines S, Lazarus C, Callister R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI) Dysphagia. 2013 Sep;28(3):350–369. doi: 10.1007/s00455-013-9451-3. [DOI] [PubMed] [Google Scholar]
- 30.Stierwalt JAG, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007 May;16(2):148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- 31.IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 32.Logemann JA, Smith CH, Pauloski BR, Rademaker AW, Lazarus CL, Colangelo LA, Mittal B, MacCracken E, Gaziano J, Stachowiak L, Newman LA. Effects of xerostomia on perception and performance of swallow function. Head Neck. 2001 Apr;23(4):317–321. doi: 10.1002/hed.1037. [DOI] [PubMed] [Google Scholar]
- 33.Navazesh M, Ship II. Xerostomia: diagnosis and treatment. Am J Otolaryngol. 1983 Aug;4(4):283–292. doi: 10.1016/s0196-0709(83)80072-6. [DOI] [PubMed] [Google Scholar]
- 34.Levine RS. Saliva: 3. Xerostomia--aetiology and management. Dent Update. 1989 Jun;16(5):197–201. [PubMed] [Google Scholar]
- 35.Nederfors T. Xerostomia and hyposalivation. Adv Dent Res. 2000 Dec;14:48–56. doi: 10.1177/08959374000140010701. [DOI] [PubMed] [Google Scholar]
- 36.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001 Jul;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 37.Pow EHN, Kwong DLW, McMillan AS, Wong MCM, Sham JST, Leung LHT, Leung WK. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006 Nov;66(4):981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Hochberg MC, Tielsch J, Munoz B, Bandeen-Roche K, West SK, Schein OD. Prevalence of symptoms of dry mouth and their relationship to saliva production in community dwelling elderly: the SEE project. Salisbury Eye Evaluation. J Rheumatol. 1998 Mar;25(3):486–491. [PubMed] [Google Scholar]
- 39.Simcock R, Fallowfield L, Monson K, Solis-Trapala I, Parlour L, Langridge C, Jenkins V ARIX Steering Committee. ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Ann Oncol. 2013 Mar;24(3):776–783. doi: 10.1093/annonc/mds515. [DOI] [PubMed] [Google Scholar]
- 40.Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996 Oct;24(5):312–316. doi: 10.1111/j.1600-0528.1996.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Zhao Z, Li J. Investigation of the clinical value of total salival flow rates. Zhonghua Kou Qiang Yi Xue Za Zhi. 1998 Nov;33(6):360–362. [PubMed] [Google Scholar]
- 42.Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999 Mar;16(1):12–17. [PubMed] [Google Scholar]
- 43.Longman LP, Higham SM, Rai K, Edgar WM, Field EA. Salivary gland hypofunction in elderly patients attending a xerostomia clinic. Gerodontology. 1995 Dec;12(12):67–72. doi: 10.1111/j.1741-2358.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 44.Granot M, Nagler RM. Association between regional idiopathic neuropathy and salivary involvement as the possible mechanism for oral sensory complaints. J Pain. 2005 Sep;6(9):581–587. doi: 10.1016/j.jpain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Mittal B, Gaziano J, Stachowiak L, MacCracken E, Newman LA. Xerostomia: 12-month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation. Head Neck. 2003 Jun;25(6):432–437. doi: 10.1002/hed.10255. [DOI] [PubMed] [Google Scholar]
- 46.Kays SA, Hind JA, Gangnon RE, Robbins J. Effects of dining on tongue endurance and swallowing-related outcomes. J Speech Lang Hear Res. 2010 Aug;53(4):898–907. doi: 10.1044/1092-4388(2009/09-0048). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001 Oct;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 48.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, Nieman DC, Swain DP American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011 Jul;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 49.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000 Nov;55(11):M634–640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 50.Youmans SR, Youmans GL, Stierwalt JAG. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009 Mar;24(1):57–65. doi: 10.1007/s00455-008-9171-2. [DOI] [PubMed] [Google Scholar]
- 51.Youmans SR, Stierwalt JAG. Measures of tongue function related to normal swallowing. Dysphagia. 2006 Apr;21(2):102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- 52.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Gaziano J, Stachowiak L, Newman L, MacCracken E, Santa D, Mittal B. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008 Feb;30(2):148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994 Apr;37(2):314–325. doi: 10.1044/jshr.3702.314. [DOI] [PubMed] [Google Scholar]