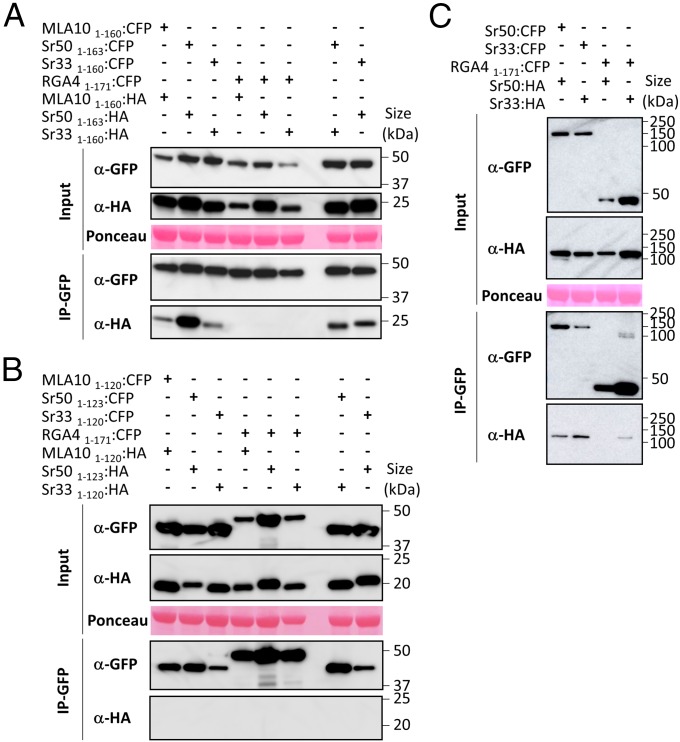
Fig. 2.
The full CC domains of Sr33, Sr50, and MLA10 can self-associate in planta. (A) The full CC domains of MLA10, Sr33, and Sr50 fused to CFP or HA tags were transiently expressed in N. benthamiana leaves in the indicated combinations (+, agro-infiltrated construct; −, non–agro-infiltrated construct), and proteins were extracted after 24 h. Tagged proteins were detected in the extract (input) and after immunoprecipitation with anti-GFP beads (IP-GFP) by immunoblotting with anti-HA (α-HA) and anti-GFP (α-GFP) antibodies. The RGA4 CC fused to CFP was used as a control for specificity. Protein loading in the input is shown by Ponceau staining of the large RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) subunit. The experiment was carried out three times with identical results. (B) The truncated CC domains of MLA10, Sr33, and Sr50 fused to CFP or HA were transiently expressed in N. benthamiana leaves in the indicated combinations. Samples were processed as described in A. The experiment was carried out twice with identical results. (C) The procedure described in A was applied to the full-length Sr33 and Sr50 proteins.