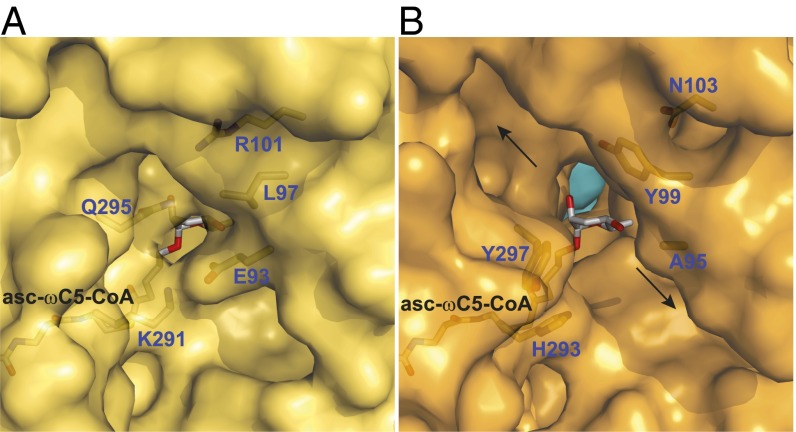
Fig. 3.
Amino acid residues that determine whether the active site is closed or open to the external surface. (A) A region of the outer surface of ACOX-2(E432A) (yellow), showing that its bound substrate asc-ωC5-CoA (2) is largely encased by the protein. The FAD cofactor located behind the substrate is not shown for clarity. A hydrogen bond between Gln-295 and Arg-101 and a salt bridge between Lys-291 and Glu-93 close the active site to the outer surface. (B) A region of the outer surface of ACOX-1(E434A) (orange, with the other subunit in light blue). The substrate of ACOX-2 (asc-ωC5-CoA, 2) has been placed into the ACOX-1 active site to show that the active site is open to the outer surface. The FAD cofactor is not shown for clarity. Hydrophobic and aromatic residues (Ala-95, Tyr-99, His-293, and Tyr-297) line the opening to the outer surface, which leads to two channels (marked by arrows) that run along the outer surface. The location of the region shown in A and B on the full structures is shown in SI Appendix, Fig. S5 A and B.