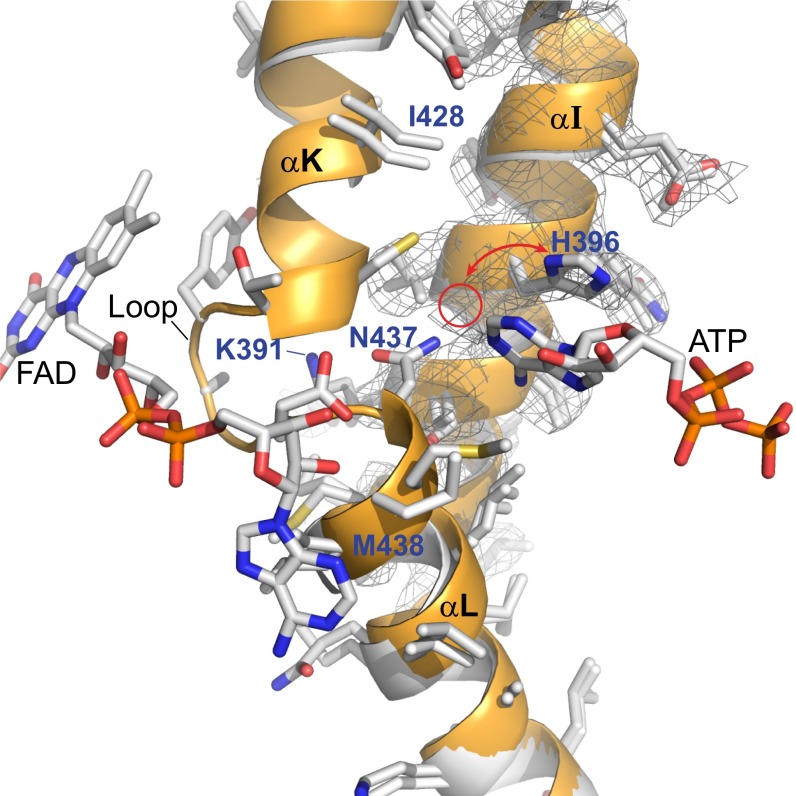
Fig. 6.
Interaction between the ATP and FAD binding sites. Overlay of apo-ACOX-1 structure (no bound ATP or FAD) in white with holo-ACOX-1(E434A) structure (with bound ATP and FAD) in orange. α-helices αI, αK, and αL, as well as the electron density (2Fo-Fc map, σ=1.0) associated with αI of apo-ACOX-1, are shown. The loop between αK and αL is disordered between Ile-428 and Met-438 in apo-ACOX-1 and is ordered in ACOX-1(E434A) and interacts with FAD. The electron density of the apo-ACOX-1 structure shows that His-396 can adopt two different conformations, one of which is shown and one of which is indicated with a red circle. The back side of this image is shown in SI Appendix, Fig. S11, and a stereoview of this image and its back side is shown in SI Appendix, Fig. S12 A and B.