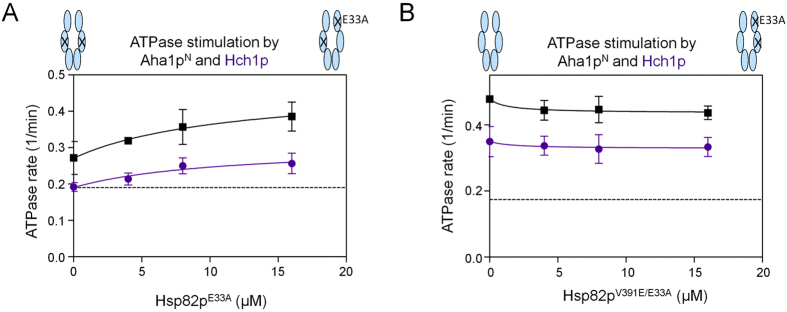
Figure 5. N-terminal Aha1p-type co-chaperones stimulate Hsp82p ATPase activity from either catalytic or non-catalytic protomer.
(A) Hch1p- and Aha1pN-mediated ATPase stimulation of Hsp82pV391E:Hsp82pE33A heterodimers. Reactions contained 4 μM Hsp82pV391E, 20 μM Hch1p (purple) or Aha1pN (black), and indicated concentrations of Hsp82pE33A. Intrinsic rate of Hsp82pV391E shown as black stippled line. (B) Hch1p- and Aha1pN-mediated ATPase stimulation of Hsp82p:Hsp82pV391E/E33A heterodimers. Reactions contained 4 μM Hsp82p, 20 μM Hch1p (purple) or Aha1pN (black), and indicated concentrations of Hsp82pV391E/E33A. Intrinsic rate of wildtype Hsp82p shown as black stippled line. ATPase rates are shown in micromolar ATP hydrolyzed per minute per micromolar of enzyme (1/min).