Figure 2.
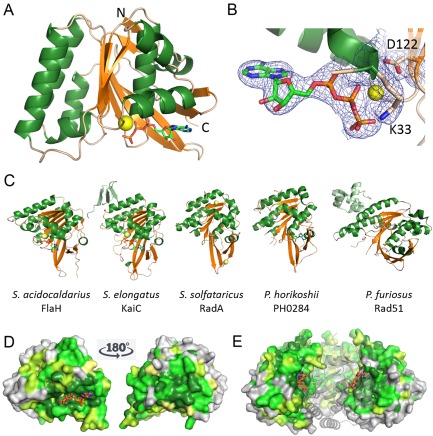
Crystal structure of FlaH with ATP showing fold, ATP binding and conserved surface regions.
A. Ribbon representation of the S . acidocaldarius FlaH monomer showing fold, secondary structure and ATP binding site. ATP (sticks) bind to the active site and Mg2+ ion (yellow sphere). N‐ and C‐termini are indicated.
B. Active site with electron density (2|Fo|‐|Fc| map at 1σ) for the bound ATP and Mg2+‐ion. The Walker A and Walker B residues K33 and D122 are indicated.
C. FlaH compared with other RecA superfamily ATPases. Similar structures revealed by a DALI search for structural homologues are shown. Additional subunits besides the RecA/Rad51 domain are shown in light green. Nucleotides or phosphate bound to the active sites are shown in sticks, ions as spheres.
D. Conservation of residues is depicted on the SaFlaH surface. Dark green and light green indicate identical and similar residues respectively. FlaH monomers are in the same orientation as in A and turned 180° and shown as surface representation. ATP is shown as orange sticks. Mg2+ ion as a yellow sphere.
E. FlaH interface within the hexamer modeled by superposition on the PH0284 hexamer: one monomer shown as cartoon to visualize the highly conserved interfaces. ATP is shown as orange sticks. Mg2+ ion as yellow sphere.
