Figure 5.
SaFlaH interacts with SaFlax and forms substructures inside the SaFlaX ring in vitro.
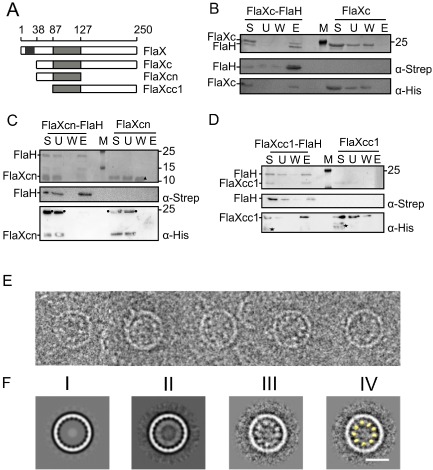
A. A schematic of SaFlaX truncates used in this study. The black box indicates the membrane domain (residues 10–32), and the light gray box is the coiled coil region (residues 87–127) of SaFlaX.
B. StrepII‐tagged SaFlaH and His6‐tagged SaFlaXc were mixed (S, starting material) and incubated with streptactin beads. Unbound proteins were removed (U, unbound fraction), the beads were washed (W, wash fraction) and proteins were eluted with 5 mM d‐desthiobiotin (E, elution). As a control, a similar experiment was performed in the absence of SaFlaH. Fractions were separated on a SDS‐PAGE and visualized by Coomassie staining. Fractions were also transferred after SDS‐PAGE to PVDF membranes, and proteins were detected using anti‐Strep and anti‐His antibodies. M depicts the PAGE size standard. An analogous interaction experiment was performed with His6‐tagged SaFlaXcn (C), where no interaction with FlaH was observed. In contrast SaFlaXcc1 (D) showed interaction with FlaH. These results suggest that the C‐terminal domain of FlaX is essential for the interaction with FlaH. Consistent with Banerjee et al. (2012), an asterisk (*) depicts degraded protein in the case of FlaXcc1 in D, a circle (●) depicts an oligomeric forms of the FlaXcn as visualized in the anti‐His blot, a filled triangle (Δ) depict monomers of FlaXcn visualized on SDS‐PAGE.
E. Cryo‐EM images of FlaX/FlaH complexes: selected rings belonging to the class with 20‐fold symmetry. Discrete particles are visible inside the rings, probably representing SaFlaH.
F. Class averages of ring images. (I) SaFlaX ring with 20‐fold symmetry (Banerjee et al., 2012); (II) 20‐fold symmetric SaFlaX/SaFlaH complexes aligned to the SaFlaX ring; (III) Particles belonging to the class average in II aligned to the inner part of the ring. Discrete densities are visible; these densities were smeared out in II resulting in a continuous inner ring; (IV) The densities inside the SaFlaX rings correspond to the size and shape of the monomer structure of SaFlaH (yellow, filtered to 40 Å resolution). Scale bar = 20 nm.
