Figure 6.
Proposed model of FlaH binding within the FlaX ring and nucleotide‐regulated FlaH binding to FlaI to form the archaellar basal body core.
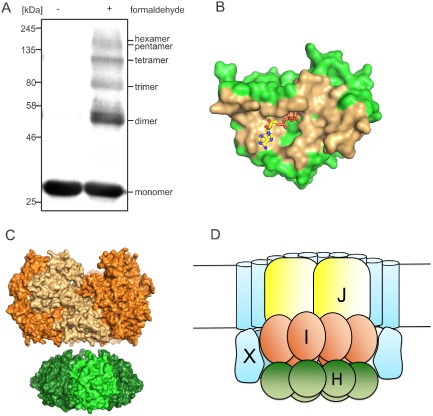
A. FlaH oligomerization by cross‐linking. FlaH was incubated with or without formaldehyde, separated on SDS‐PAGE and detected by anti His‐tag antibody.
B. FlaH residues involved in interface formation are colored orange for the FlaH‐FlaH interaction modeled by comparison with the PH0284 hexamer.
C. Side view of the SaFlaH hexamer (green) modeled based on the hexameric PH0284 of P . horikoshii with SaFlaI (orange) illustrate that the outer diameters of both protein oligomers are of comparable size.
D. Cartoon of our proposed quaternary structure of the archaellum motor complex taking into account previous data, the cross‐linking result and the FlaH‐FlaX localization and interaction data from Fig. 5.
