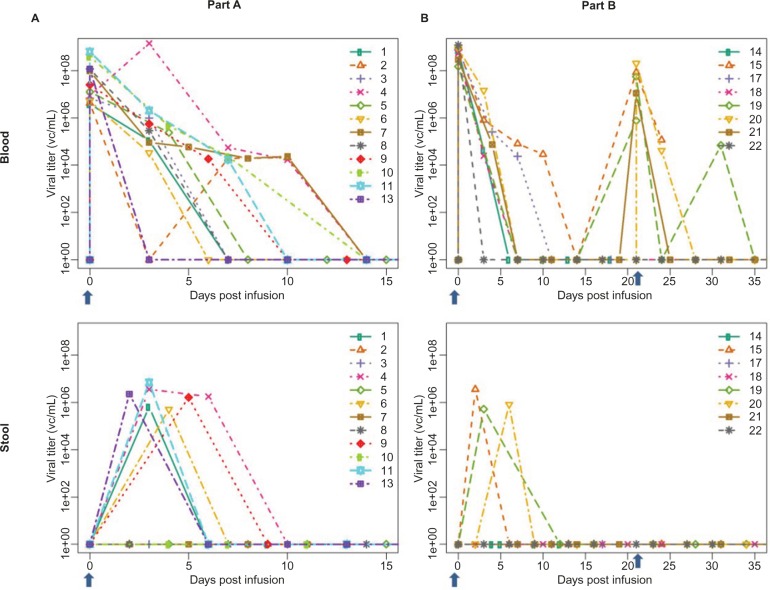
Figure 4.
Viral clearance of SVV-001.
Notes: Viral clearance in the blood and stool over time after a single dose (A) and after two doses (B) of SVV-001 in the COG ADVL0911 pediatric trial. The arrow denotes timing of SVV-001 administration. Reproduced with permission from John Wiley and Sons. Burke MJ, Ahern C, Weigel BJ, et al. Phase I trial of Seneca Valley virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(5):743–750.16 © 2015 John Wiley and Sons.
Abbreviations: SVV-001, Seneca Valley Virus isolate 001; COG, Children’s Oncology Group.