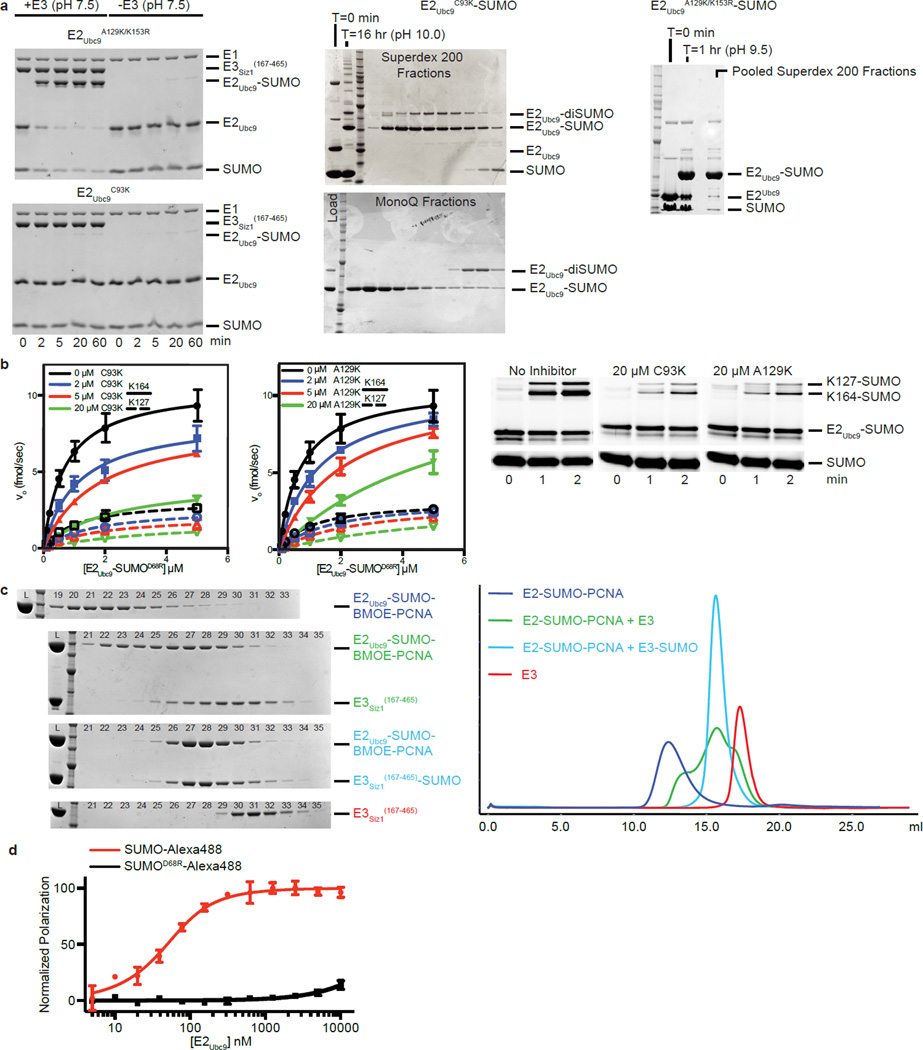
Extended Data Figure 1. E2Ubc9-SUMO Thioester Mimic and Cross-Linking to Substrate PCNA for Reconstitution With E3Siz1.
a, SDS-PAGE analysis of in vitro E2Ubc9A129K or E2Ubc9C93K charging with SUMO in the presence and absence of E3Siz1(167–465) at pH (7.5) (left) and purification of the E2Ubc9C93K-SUMO (middle) and E2Ubc9A129K-SUMO (right) thioester mimetics. b, Plots of rates for in vitro SUMO modification of PCNA in assays utilizing various concentrations of purified E2Ubc9-SUMOD68R-Alexa488 labeled thioester, 1 nM E3Siz1(167–465) and 32 µM PCNA with 0, 2, 5 or 20 µM of the E2Ubc9C93K-SUMO or E2Ubc9A129K-SUMO thioester mimic (left) with exemplary non-reducing SDS-PAGE for the 0.5 µM E2Ubc9-SUMOD68R-Alexa488 reactions (right). The calculated Km and Ki from these fits are shown in Extended Data Tables 1a and 2 and the quantified data show mean ± s.d. (n=3 technical replicates). c, SDS-PAGE analysis (left) of numbered 0.5 ml fractions from Superose6 analytical gel-filtration analysis (right) of complex reconstitution between E2Ubc9-SUMO-BMOE-PCNA and E3Siz1(167–465) (green) or the E3Siz1(167–465)-SUMO fusion (blue). Elution profiles for E2Ubc9-SUMO-BMOE-PCNA (purple) and E3Siz1(167–465) (red) alone are shown. d, Plot of the normalized change in polarization observed upon addition of serially diluted E2Ubc9 with Alexa488 labeled SUMO or SUMOD68R. Data were fit to single-site binding model accounting for receptor depletion. Data show mean ± s.d. (n=3 technical replicates). For gel source data, see Supplementary Figure 1.