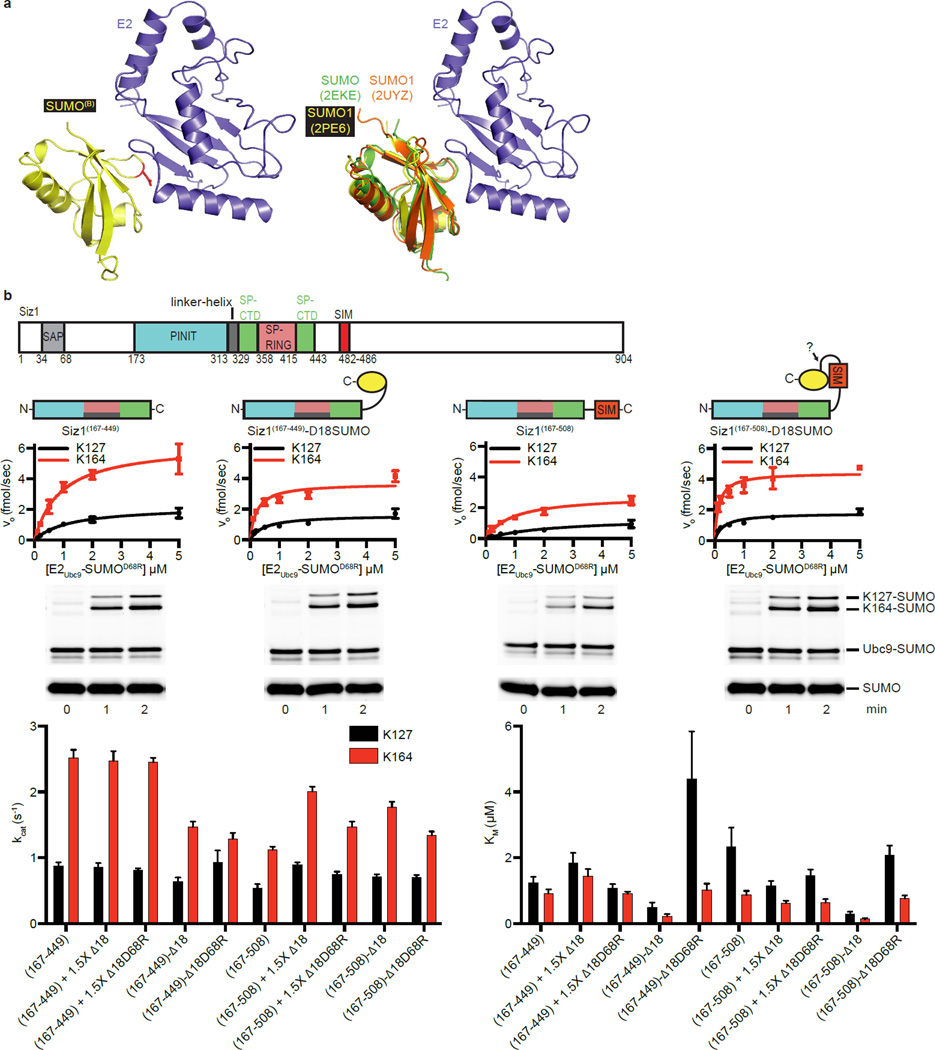
Extended Data Figure 4. SUMOB Bound to the E2 Backside enhances E2Ubc9-SUMO Recruitment.
a, Alignment of the current E2Ubc9/backside SUMOB (left) to previously observed E2Ubc9/backside SUMO complexes (right). The position of the D68R mutation is shown in red sticks (left). b, Primary E3Siz1 structure (top). Cartoons indicating the E3Siz1 or E3Siz1-SUMO fusion constructs utilized in the multiple turnover in vitro assays (middle) shown in Fig. 3 utilizing a titration of the purified E2Ubc9-SUMOD68R-Alexa488 thioester with or without 1.5-fold excess of the indicated additional molecule of non-conjugatable SUMO, 1 nM of the indicated E3 construct and 32 µM PCNA. Representative non-reducing SDS-PAGE showing the 0.5 µM E2Ubc9-SUMOD68R-Alexa488 thioester reactions below the plots of the rates of reaction for each E2Ubc9-SUMOD68R concentration (middle). The kinetics of SUMO modification of PCNA were calculated and the Km and kcat were determined (bottom) and are also shown in Extended Data Table 2. The quantified rate data show mean ± s.d. (n=3 technical replicates). For gel source data, see Supplementary Figure 1.