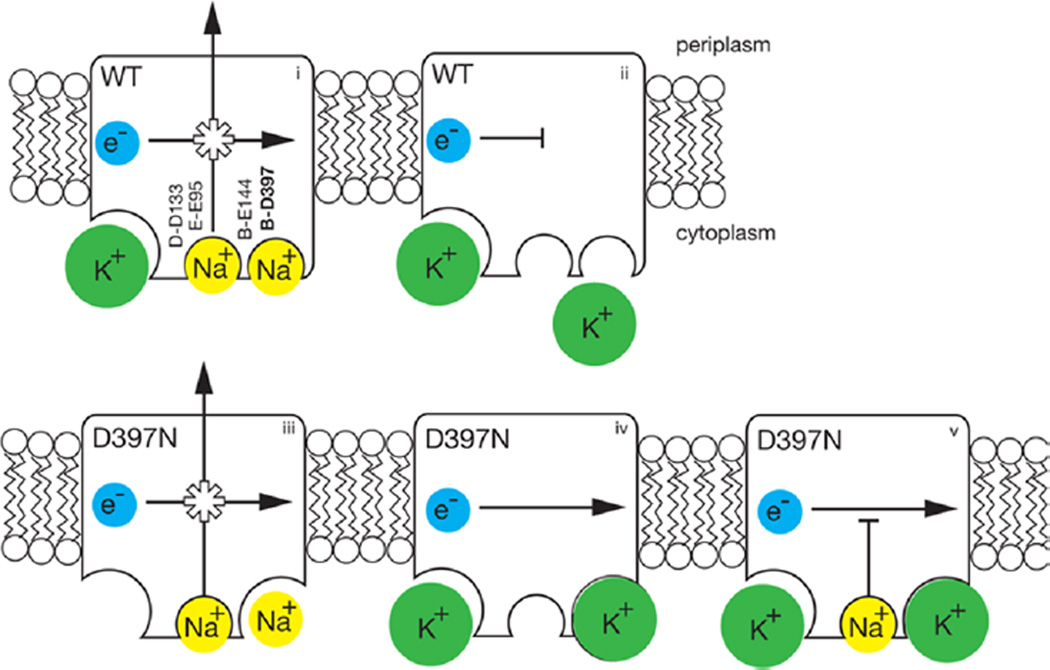
Figure 5.
Scheme illustrating properties of the NqrB-D397N mutant compared to those of the wild-type enzyme. In red are shown the acidic residues involved in Na+ uptake. In the wild-type enzyme (top row, left to right), (i) electron flow is coupled to Na+ pumping, K+ binds to a different, allosteric site (bottom left corner) and accelerates the reaction (ii) in the absence of Na+, and electron flow is inhibited, confirming that the redox and pumping processes are coupled. In the NqrB-D397N mutant (bottom row, left to right), (iii) in the absence of K+, electron flow is still coupled to Na+ pumping; (iv) in the presence of K+ (in the absence of Na+), electron flow proceeds, but without ion pumping, meaning that coupling has been disrupted; and (v) K+ causes the mutant to run uncoupled, even in the presence of Na+. Of the acidic residues involved in cation uptake, shown in bold, only NqrB-D397 is associated with the modified binding site.