Abstract
Neurosensory and behavioural disruptions are some of the most consistently reported responses upon exposure to ocean acidification-relevant CO2 levels, especially in coral reef fishes. The underlying cause of these disruptions is thought to be altered current across the GABAA receptor in neuronal cells due to changes in ion gradients (HCO3− and/or Cl−) that occur in the body following compensation for elevated ambient CO2. Despite these widely-documented behavioural disruptions, the present study is the first to pair a behavioural assay with measurements of relevant intracellular and extracellular acid-base parameters in a coral reef fish exposed to elevated CO2. Spiny damselfish (Acanthochromis polyacanthus) exposed to 1900 μatm CO2 for 4 days exhibited significantly increased intracellular and extracellular HCO3− concentrations and elevated brain pHi compared to control fish, providing evidence of CO2 compensation. As expected, high CO2 exposed damselfish spent significantly more time in a chemical alarm cue (CAC) than control fish, supporting a potential link between behavioural disruption and CO2 compensation. Using HCO3− measurements from the damselfish, the reversal potential for GABAA (EGABA) was calculated, illustrating that biophysical properties of the brain during CO2 compensation could change GABAA receptor function and account for the behavioural disturbances noted during exposure to elevated CO2.
Concerns about the impact of ocean acidification on marine ecosystems has led to a growing number of studies examining the effects of elevated CO2 exposure on fish1. While some investigated endpoints such as survival and growth appear to be relatively insensitive to projected future CO2 levels2,3, significant effects of elevated CO2 include alterations to mitochondrial function4,5, metabolic rate6, otolith growth7,8, reproduction9,10, and acid-base balance11,12. Perhaps the most frequently reported and consistently adverse response to elevated CO2 exposure in fish is disruption to sensory or cognitive function. Impairments to olfaction13,14,15, hearing16, vision17,18, lateralization19,20,21, and learning22,23 in fish at ocean acidification relevant CO2 levels demonstrate that CO2 broadly affects central neuronal processing. Neurosensory impacts are particularly concerning since these traits appear to show limited capacity for acclimation13. Furthermore, fish living near highly acidic natural CO2 vent systems that presumably experience high CO2 on a regular basis also exhibit abnormal behavioural responses24. Considering the rapid rate of acidification25 and the low CO2 threshold level needed to induce sensory and neurological responses (~600–800 μatm CO2)26, understanding the physiological mechanism underlying these responses is crucial for assessing risk to fish populations and could aid in predicting adaptive capacity.
Most studies to date suggest that significant effects of CO2, including behavioural disturbances, result from compensation that fish perform in response to a CO2-induced respiratory acidosis. Following exposure to elevated CO2, fish correct plasma and tissue pH by sustaining elevated HCO3− levels in intracellular and extracellular fluids12,27,28,29. Although pH is corrected to pre-exposure levels, HCO3− and PCO2 consequently remain elevated throughout high CO2 exposure. Increased plasma HCO3− concentrations are often paired with a corresponding decline in Cl− 27,30,31. Surprisingly, examination of CO2 acid-base balance disturbances and associated compensatory mechanisms have only been performed at ocean acidification relevant scenarios in a limited number of studies5,12,32,33.
In 2012, Nilsson and colleagues reported a series of seminal experiments on fish, suggesting compensation for elevated CO2 affects olfaction and lateralization by disrupting the function of the GABAA receptor34. Under most circumstances, the GABAA receptor and its associated neurotransmitter (GABA) are thought to be largely responsible for inhibitory responses throughout the vertebrate nervous system. In the model proposed by Nilsson and colleagues, following stimulation by GABA, HCO3− and/or Cl− ions enter the cell through the GABAA receptor under control conditions, leading to cellular hyperpolarization, and a concomitant inhibitory response that is associated with a normal behavioural phenotype in fish. However, expected changes in extracellular and/or intracellular HCO3− and Cl− that occur during CO2 compensation are thought to reverse ion movement through the GABAA receptor, leading to a depolarizing excitatory response and a disrupted behavioural phenotype34. Alleviation of olfactory and lateralization disturbances in CO2-exposed fish upon treatment with gabazine, a competitive GABAA receptor antagonist that presumably closes the GABAA receptor, implicated GABAA receptor involvement in the impaired behavioural responses induced by elevated CO2. Since this initial study, the apparent link between CO2-induced behavioural disturbances and the GABAA receptor has been supported by several other studies examining a variety of species (tropical and temperate, marine and freshwater), utilizing an array of sensory and behavioural assays as well as different GABAA receptor antagonists and agonists17,21,22,35,36,37,38. Similar behavioural effects of high CO2 exposure that are restored by GABAA receptor antagonists have also been observed in marine invertebrates39. Further support for the role of the GABAA receptor in abnormal behaviour during CO2 exposure has been provided by theoretical calculations of the GABAA receptor equilibrium potential (EGABA)1 using HCO3− values estimated from the Gulf toadfish12. However, it is important to keep in mind that altered ion gradients due to high CO2 exposure would not necessarily have to cause a complete reversal of current to invoke a behavioural change. Even an attenuation of the normal inhibitory response of the GABAA receptor due to changes in ion gradients could alter the function of neurons and account for noted behavioural disruptions.
Behavioural assays paired with GABAA-targeted drug treatments have strongly supported the argument that altered ion gradients in a high CO2 environment change the function of the GABAA receptor; however, adjustments to acid-base parameters that would reverse or attenuate the current through the GABAA receptor have yet to be measured in a marine fish showing a behavioural disruption. Accordingly, the aim of this study was to test the hypothesis that altered intracellular and extracellular HCO3− due to CO2 compensation occurs in a species that also exhibits a behavioural disturbance when exposed to elevated CO2. The first objective of this study was to measure intracellular whole-brain HCO3− and pH (pHi) and extracellular HCO3− levels in blood plasma of the spiny damselfish (Acanthochromis polyacanthus) exposed to either control or 1900 μatm CO2. A second objective was to confirm that spiny damselfish exposed to the applied CO2 level displayed altered behavioural responses to olfactory cues as previously reported13,15. The third and final objective was to apply the measured values in an assessment of GABAA receptor function by calculating EGABA in control and CO2-exposed fish. To our knowledge, this is the first study to report direct measurements of both intracellular and extracellular HCO3− and intracellular pHi in a coral reef fish species.
Results
Physiological measurements: Brain and plasma analyses
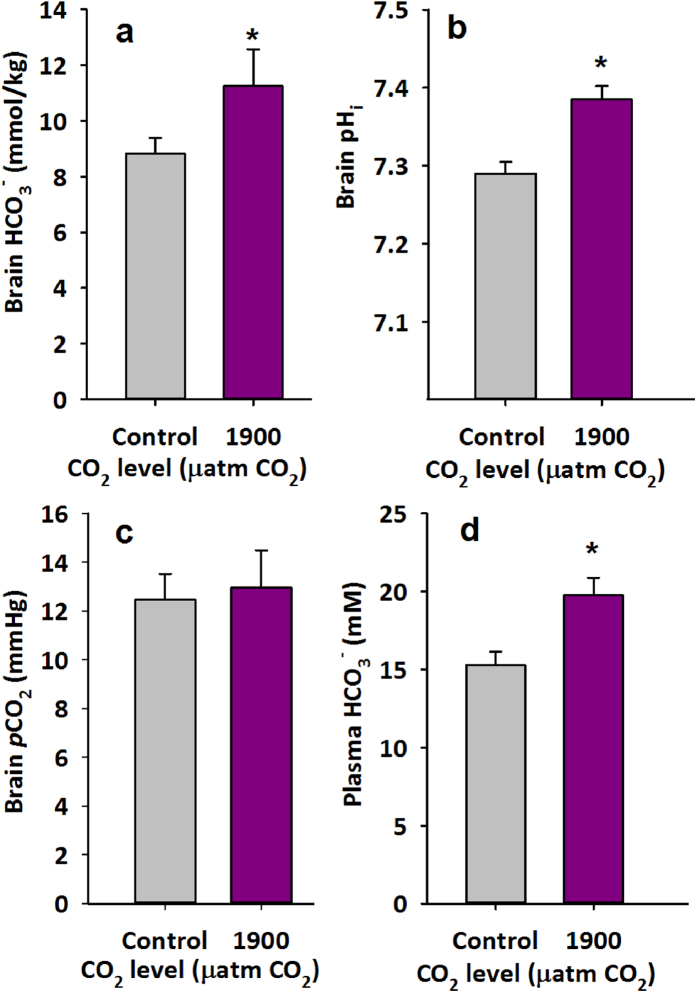
As expected, brain HCO3− (mmol/kg) and brain pHi (Fig. 1a,b) were both significantly higher in damselfish exposed to 1900 μatm CO2 for 4 days when compared to controls (Fig. 1a,b; brain HCO3−, P < 0.03, brain pHi, P < 0.001). Using these values and pK’ and solubility constants from Boutilier and colleagues40, the brain PCO2 was calculated using the Henderson-Hasselbalch equation and displayed no significant difference between control and CO2-exposed fish (Fig. 1c). Plasma HCO3− (mM) was also significantly higher in CO2-exposed fish when compared to controls (P < 0.008, Fig. 1d). Due to low blood volumes and small fish size, low plasma volume precluded measurements of pH, preventing PCO2 calculations for extracellular fluids. In order to verify the high levels of HCO3− found for both brain and plasma readings a series of validation procedures were performed (Fig. S1). Measurements of a series of blanks (NaCl solution only, 50 mM) and bicarbonate standards in the range of values measured in the brain and plasma samples indicated near perfect agreement between expected and measured values. HCO3− standards were made by diluting a 10 mmol l−1 NaHCO3− solution into the NaCl solution used for determination of blank/background levels. An observed, low level of background HCO3−, represented by the constant offset from a predicted 1:1 slope, is shown in Supplementary Fig. S1. This offset was nearly negligible (60 nmol) but was nonetheless subtracted from all reported values.
Figure 1.
Brain and plasma analysis in the spiny damselfish exposed to high CO2: (a) Brain HCO3− (mmol/kg tissue), (b) intracellular pH and C) PCO2 (Means ± s.e.m.; n = 8 for PCO2 and n = 8 and n = 6 for control and 1900 μatm CO2, respectively for HCO3− and intracellular pH) in spiny damselfish (Acanthochromis polyacanthus) exposed to either control or 1900 μatm CO2 for 4 days. D) Plasma HCO3− (mM) (Means ± s.e.m.; N = 7) of spiny damselfish (Acanthochromis polyacanthus) exposed to either control (value) or 1900 μatm CO2 for 4 days. *Denotes statistical significance from respective control value at P < 0.05. Brain HCO3− was assessed with one-tailed t-test. N = 8 for PCO2 and n = 8 and n = 6 for control and 1900 μatm CO2, respectively for HCO3− and intracellular pH.
Behavioural response to a chemical alarm cue
Fish that were maintained in the laboratory at 1900 μatm CO2 for 4 days, when tested in a two-choice flume chamber, spent approximately half (53%) of their time in conspecific chemical alarm cue rather than untreated water. In contrast, control fish spent only ~15% in the CAC (Fig. 2, P < 0.001).
Figure 2. Response to alarm cues under high CO2 in the spiny damselfish: Percent time (mean ± s.e.m) spent in chemical alarm cue using a two-flume choice chamber for spiny damselfish (Acanthochromis polyacanthus) exposed to control or 1900 μatm CO2 for 4 days.
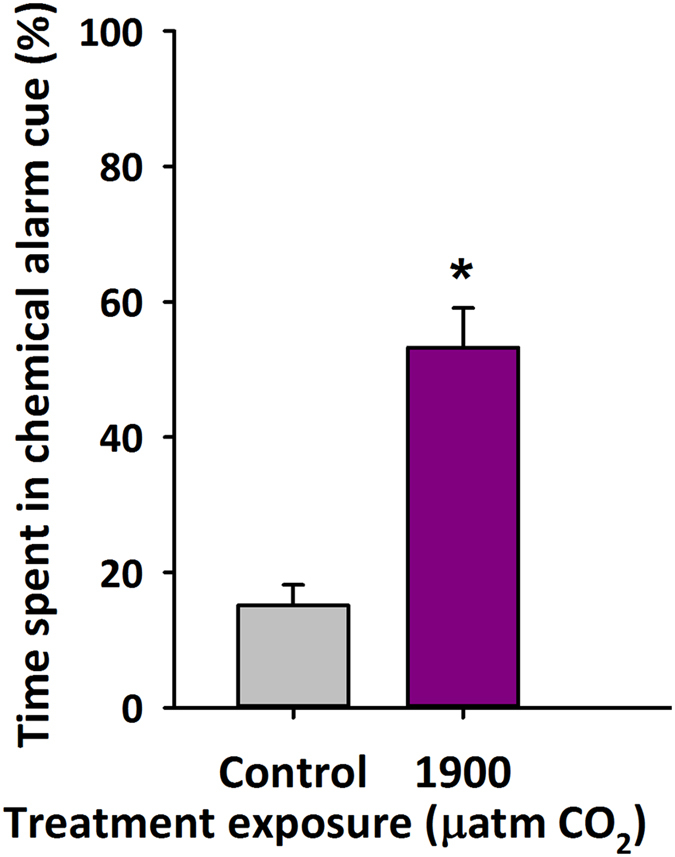
Fish (N = 20) were offered either control water or water containing a chemical alarm cue in the choice chamber. *Denotes statistical significance from respective control value at P < 0.05.
Discussion
As expected, our results show that spiny damselfish, compensate for a CO2 induced acidosis by elevating plasma and brain HCO3− following exposure to 1900 μatm CO2 for 4 days (Fig. 1). Also as predicted, this compensatory response appears to be associated with a reduction in chemical alarm cue avoidance behaviours (Fig. 2), suggesting impaired olfaction and/or central neuronal processing. The magnitude of HCO3− change between control and CO2 exposed fish (2.4 mM) compares to that found in white muscle in both the rockcod (2.7 mM)5 and the toadfish (3.2 mM)1,12 at similar CO2 levels (1900–2000 μatm CO2). Control measurements of brain HCO3− (8.8 mM) were at the high end of ranges calculated or measured for tissues in other species (0.5–9.2 mM)5,41,42,43. As evident from the equation below, the relative rather than absolute changes in ions both inside and outside the cell are relevant in assessing whether or not CO2 compensation alters the function of the GABAA receptor. Accordingly, measurements of plasma HCO3− were also obtained, allowing for better assessment of gradients across neuronal cell membranes. The relative difference in plasma HCO3− concentrations between control and CO2 exposed fish (~4.5 mM HCO3−) was similar to that seen in toadfish (∆3.3 mM; 1900 μatm CO2)12, marbled rockcod (∆3.2 mM; 2000 μatm CO2)5, spotted catshark (∆3.0 mM; 1000 μatm CO2)32, red drum (~∆2.0 mM; 1000 μatm CO2)33, and the epaulette shark (~∆2.0 mM; 880 μatm CO2)44. However, the absolute HCO3− levels for both control (15.3 mM) and 1900 μatm CO2 (19.8 mM) exposed damselfish were high compared to values reported in other species, ranging from ~3 mM up to ~11.3 mM in control animals5,12,29,30,31,33,41,42,43,44,45,46. Titration of known standards showed high accuracy. Therefore, other explanations for the high plasma HCO3− concentrations may include the blood sampling procedure used. Ideally, blood samples for consideration of acid-base balance parameters should be taken from cannulated resting and unstressed fish under gastight conditions, an option that was unavailable in the present study due to the small size of the damselfish. Rather, blood was obtained by caudal puncture. Potential errors associated with caudal puncture can result from fish being anesthetized and briefly air-exposed during sampling, preventing CO2 excretion, and could lead to an overestimation of plasma HCO3− concentrations47. However, a recent study using red drum (Sciaenops oceallatus) comparing plasma samples obtained from cannulated fish and those obtained by caudal puncture revealed the similar CO2-induced increase in HCO3−. Furthermore, the comparisons revealed that caudal puncture caused an increase of about 1.5 mM or ~20% HCO3− in plasma HCO3− compared to values obtained from catheters33. On balance, the levels reported here may overestimate true plasma HCO3− levels in damselfish. Nonetheless, the increase in plasma HCO3− observed in response to CO2 is a product of ambient conditions, since sampling procedures were identical for control and CO2-exposed fish. Brain HCO3− measurements in the present study were not associated with the same potential errors inherent with plasma measurements but were also at the high end of reported ranges for tissue HCO3− in other species.
Interestingly, pHi in the brain of high CO2-exposed fish was significantly higher than in control fish (∆0.095), demonstrating a pHi overshoot, a response common across many organs, species, and CO2 levels5,12,48. In the limited number of studies measuring intracellular pH at similar CO2 levels, white muscle and liver of the marbled rockcod show compensation with no overshoot (2000 μatm CO2)5, while the white muscle of the Gulf toadfish exhibit a pHi overshoot of a similar magnitude (~∆0.07; 1900 μatm CO2)12 as seen in the damselfish brain. As suggested in Esbaugh et al.12, such an overshoot could result from active intracellular regulation to take up HCO3− from extracellular fluids or could merely reflect passive uptake due to higher HCO3− levels in extracellular fluids. Given the relatively high levels of plasma HCO3− reported in this study, either explanation seems possible. Regardless of the underlying cause, it is clear that HCO3− availability is not a limiting factor for intracellular compensation for elevated CO2. Further investigation into the downstream impacts of pHi protection during CO2 exposure is needed to fully understand the tradeoffs between acid-base and neuronal homeostasis. Interestingly, the overshoot of pHi in damselfish brains means that estimated PCO2 levels were not significantly elevated despite elevated ambient CO2 and elevated intracellular HCO3− concentrations (Fig. 1).
Measurements in the present study, the first of their kind, showing altered intracellular and extracellular HCO3− in a marine species that also exhibits a CO2-induced disturbance to olfactory-mediated behaviour lends support to the hypothesis that altered ion gradients can affect GABAA receptor function34. These direct measurements also, also for the first time, provide the advantage of allowing for calculation of the reversal potential for the GABAA (EGABA) under control conditions and high CO2 conditions. The addition of gabazine (GABAA receptor antagonist) has alleviated CO2 induced behavioral impairments in many species to date17,21,22,34,36,37,38, suggesting GABAA is impacted during CO2 exposure. Thus, results from these calculations lend support to previous reports of behavioural disturbances. One caveat to this approach, is that GABAA receptor function was not directly tested in the present study, as previous studies have done primarily using the addition of gabazine. However, gabazine has been found to attenuate impairment to retinal function in this study species17, and has alleviated behavioral impairments in closely related damselfish species studied to date22,34.
Modeled after calculations described previously (Heuer and Grosell 2014), EGABA was calculated using the following equation49:
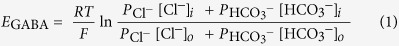 |
where R is the ideal gas constant, T is the absolute temperature, F is Faraday’s constant, and P represents the relative permeability of the GABAA receptor for HCO3− and Cl−. Intracellular and extracellular values for HCO3− (Fig. 1.) were used to calculate EGABA for damselfish. Extracellular Cl− was assumed to be 150 mM, a typical value for marine teleosts. Under high CO2 conditions, HCO3− is generally assumed to increase in extracellular fluids with a corresponding decrease in Cl− 27,30,31. Thus, the increase in HCO3− in high CO2 was used to adjust extracellular Cl−. Intracellular Cl− was chosen to be 8 mM, within the range of values reported from a recent review (6–14 mM)50. GABAA exhibits conductance for both HCO3− and Cl− in the physiological range, but tends to be more permeable to Cl− 51. Different permeability ratios (P) have been measured in neurons in invertebrates and mammals ranging from ~0.18–0.649. Since values have not been reported for fish, EGABA was calculated over a representative range of permeability ratios (0.2–0.5) (Fig. 3). All input variables applied in the calculations presented in Fig. 3 are summarized in Supplementary Table S2.
Figure 3. Calculated EGABA values based on equation (1) over a range of physiologically relevant HCO3−/Cl− permeability ratios for the GABAA receptor in the spiny damselfish and the Gulf toadfish: For damselfish, brain and plasma HCO3− concentrations from Figs 1 and 2 were used for [HCO3−]i (intracellular) and [HCO3−]o (extracellular), respectively.
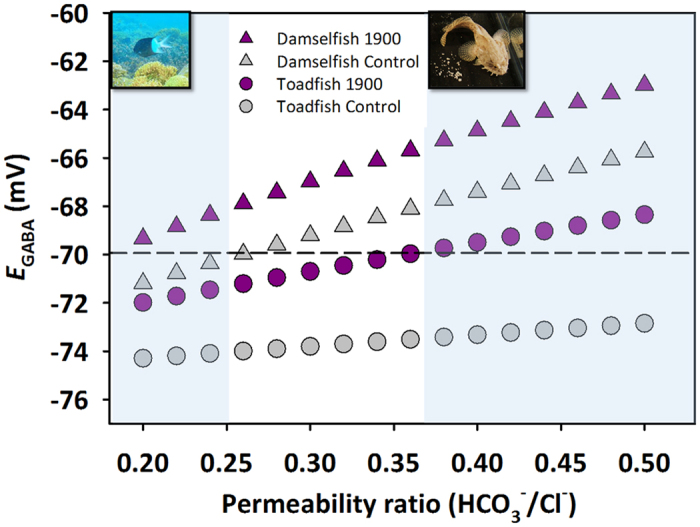
Toadfish values were taken from12 and modeled after calculations in1 using [Cl−]i of 8 mM50. Permeability ratios were chosen to represent a range of values previously reported to be physiologically relevant; see review49. For both species, values for extracellular Cl− were assumed to be 150 mM65, a typical value for marine teleosts and was adjusted assuming equimolar exchange of HCO3− and Cl− between extracellular fluids and the environment that has been demonstrated to occur during CO2 exposure in other teleosts30,31. Intracellular [Cl−] was assumed to be 8 mM50. Study temperatures of 27 °C and 25 °C were used for the calculations for damselfish and toadfish, respectively. A standard resting neuronal membrane potential of −70 mV was used to assess divergence from resting. Shaded areas represent the range of permeability ratios in which calculated EGABA diverges in opposite directions from the resting membrane potential for the species pictured. CO2-exposed fish with a calculated EGABA above the dashed line would show an abnormal depolarizing or excitatory response to GABA corresponding to an abnormal behavioural phenotype. Values used for calculations are summarized in Supplementary Table S2.
Calculated EGABA values for damselfish exposed to control and 1900 μatm CO2 conditions show a divergent deviation from the commonly assumed resting neuronal membrane potential (−70 mV) under a range of physiologically relevant permeability ratios (0.2–0.25; Fig. 3). In these instances, EGABA for control damselfish shows a negative deviation from resting, likely conferring a normal hyperpolarizing and inhibitory response. In contrast, EGABA for high CO2-exposed damselfish shows a positive deviation from resting, illustrating the potential for an abnormal depolarizing and excitatory response. In addition to damselfish, the potential for divergent responses from resting potential using calculations of EGABA using acid-base parameters has also been estimated for the Gulf toadfish1. Using calculated or measured HCO3− levels from a previous study1,12 and assuming the same [Cl−]i level (8 mM) as the damselfish, toadfish also show a divergent deviation from the resting membrane potential, however, over a higher range of permeability ratios (0.38–0.5, Fig. 3). Finally, intracellular and extracellular values of HCO3− have also been calculated for white muscle in a polar fish, the marbled rockcod (Notothenia rossii) exposed to 2000 μatm CO2. Using these measurements as a proxy for the brain, it appears that at 8 mM [Cl−]i, no divergent response would be noted between control and high CO2 exposed fish. However, if Cl− is assumed to be at a lower value in the physiological range (6 mM), divergent responses are noted over a wide range of permeability ratios (0.26–0.48; Supplementary Table S2). Thus, under a given set of physiologically relevant scenarios in tropical, subtropical, and polar fish, divergent responses of currents though GABAA are noted with ocean acidification relevant CO2 exposure levels. The above calculations and assumptions illustrate that reversal of current through the GABAA receptor may occur in fish exposed to climate change relevant CO2 levels, which may underpin the altered behavioural responses. However, it should be noted that even shifts in the degree of a hyperpolarizing current in response to GABAA rather than a full reversal could alter behaviour, as it could lead to attenuated inhibitory effects of GABA.
Although using the EGABA model in the present study is likely an oversimplification of a complex response, it may provide a useful tool in formulating hypotheses about patterns of behavioural disturbance. The temperate wrasse52 and the Atlantic cod53 are both species that do not exhibit certain behavioural alterations following CO2 exposure and would be useful to examine in this context. A testable prediction is that these species do not exhibit drastic alterations of HCO3− gradients across neuronal cell membranes during CO2 exposures where no behavioural alterations are observed. On the same note, EGABA may be useful to further investigate species that show large amounts of variation in response at a particular CO2 level. For example, olfactory responses in the damselfish (Pomacentrus wardii) exposed to 700 μatm CO214 show a large degree of variation in response among individuals. Here, individuals displaying behavioural abnormality would be predicted to have more pronounced alterations of HCO3− gradients than those displaying normal behaviour during CO2 exposure. Interestingly, the percent time spent in the chemical alarm cue (53%) at 1900 μatm CO2 was less than observed at 1000 μatm CO2 in Welch et al.13 (~80%) and also in a small number of fish behaviourally tested at 1000 μatm CO2 during the present study (84%, n = 8, data not shown). Although not tested in this study, these findings suggest that the behavioural response to altered HCO3− and pHi may be non-linear. Since the EGABA calculations are temperature-dependent, it also invites hypotheses under different climate change scenarios. For example, EGABA calculated using HCO3− measurements from the polar marbled rockcod experiencing combined temperature and CO2 stressors (7 °C) also showed divergence from resting membrane potential at 6 mM [Cl]i, but over a more narrow range of permeability ratios (0.34–0.44) than with CO2 alone (0.26–0.48) suggesting that elevated temperature may alleviate or reduce behavioural disturbances in this species (see values in Supplementary Table S2). Admittedly, use of the above framework would be strengthened with measured values for intracellular chloride and GABAA receptor permeability in fish.
In conjunction with physiological measurements demonstrating altered ion gradients, there are several other factors that would aid in fully elucidating the mechanism underlying neurological disruption in fish. The GABAA receptor can vary in subunit composition which has already been predicted to confer ion permeability differences49. At least in mammals, subunit composition can vary among brain regions49,54,55, with age54, and developmental stage54. It would be useful to know the distribution and subunit composition of GABAA receptors in fish species with noted behavioural impacts. In addition, ocean acidification could also lead to regulation of neuronal transporters and enzymes involved in HCO3− and Cl− transport and is another area of fruitful research. Identification of such regulatory responses could elucidate targeted pathways for selection under future CO2 scenarios. Finally, it is important to note that the involvement of other receptors or neural pathways in CO2-induced behavioural disturbances have yet to be explored. Future work on GABAA receptors and potentially other receptors known to mediate behavioural responses in the nervous system in isolated cells from CO2-exposed fish would aid in interpreting the mechanism underlying CO2 induced behavioural alterations.
In summary, this study is the first to demonstrate CO2 compensation using direct measurements of extracellular and intracellular HCO3− values in a coral reef species known to exhibit a behavioural disturbance. Using these measurements, calculations of EGABA demonstrate that an alteration of ion movement through the GABAA receptor under high CO2 conditions is possible, and could account for the observed behavioural changes. However, more work on the GABAA receptor distribution and function would greatly aid in detailing the underlying mechanisms associated with behavioural disturbances in high CO2 exposed fish and invertebrates. Finally, it is important to acknowledge a short acclimation period was chosen for the present study since behavioural disturbances are induced after only 4 days of exposure, providing an opportunity to examine the physiological mechanisms underlying noted behavioral responses. Identification of such mechanisms may provide insight into adaptive capacity of species. Future studies examining these endpoints across generations and over longer acclimation periods would be useful in more accurately predicting impacts to fish populations in future acidic oceans.
Materials and Methods
Fish collection and acclimation
Adult spiny chromis damselfish (Acanthochromis polyacanthus) were collected from inshore reefs at Lizard Island on the Great Barrier Reef, Australia (14°40′S, 145°28′E) in April 2015. Fish were caught by barrier netting using SCUBA, and immediately brought back to the Lizard Island Research Station where they were maintained in flow-through seawater tanks for 24 hours prior to the onset of experiments. For brain measurements, fish were 19.1 ± 2.3 and 17.0 ± 1.6 g for control and 1900 μatm CO2 exposures, respectively. Fish sampled for plasma were 16.1 ± 1.4 and 15.0 ± 1.3 g for control and 1900 μatm CO2 exposures, respectively.
Damselfish were transferred to indoor 35L tanks at either control (ambient, ~450) or 1900 μatm CO2 for 4 days, a time period previously demonstrated to induce olfactory behavioural abnormalities in other reef species14. Fish were kept on a 12:12 light:dark cycle and at a consistent temperature (~27 °C; Supplementary Table S1). Fish were held in groups of ~20 per tank prior to physiological measurements (2 replicate tanks) and 3–4 fish per tank prior to behavioural assays (6 replicate tanks), provided with PVC pipe segments for shelters, and fed daily but fasted 24 h prior to sampling. Throughout sampling, individual fish were gently netted from treatment tanks and sacrificed using 0.02 g l−1 MS-222 0.2 buffered with 0.3 g l−1 NaHCO3. Minimal chase periods (<20 sec) were necessary to obtain individual fish. All experiments adhered to approved animal care protocols and collecting guidelines and in accordance with the Australia Code of Practice for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act 2001. (General Fisheries permit 170251, Great Barrier Reef Marine Park Authority Permit G13/35909.1, James Cook University Animal Ethics Committee Regulations permits A1828 and A2089). All experimental protocols were approved by the James Cook University Animal Ethics Committee.
Seawater manipulation
Ocean seawater was pumped into two 60 L header tanks at the Lizard Island Research Station. One tank was bubbled with air and served as the control tank, while the second was gassed with CO2 to achieve ~1900 μatm CO2. A CO2-stat system (Aqua Medic AT Control System) was used to dose CO2 in to the header tank to maintain pH levels at the set-point necessary to achieve 1900 μatm CO2. Seawater from these tanks was gravity fed into experimental replicate tanks at their respective CO2 level (control or 1900 μatm CO2) where temperature (C26, Comark, Norwish UK) and pHNBS (pH calibrated to National Bureau of Standards) (SevenGo Pro, Mettler Toledo, Switzerland) were recorded daily. Seawater CO2 in treatment tanks was cross-validated using a nondispersive infrared (NDIR) sensor (GMP343, Vaisala, Helsinki, Finland) connected to a portable CO2 equilibration membrane submerged in the water56. PCO2 estimated by NDIR closely matched that estimated by carbonate chemistry (Supplementary Table S1). Seawater salinity was obtained daily from the Australian Institute of Marine Science ocean monitoring sensors deployed at Lizard Island. Water samples were collected for total alkalinity (TA) three times through the experimental period. TA was measured using Gran-titrations (Metrohm 888 Titrando Titrator Metrohm, AG, Switzerland), and referenced with certified material from Dr. A.G. Dickson (Scripps Institute of Oceanography, La Jolla, CA). Values of pHNBS, TA, salinity, and temperature were entered into CO2SYS using the constants K1 from Merhbach et al.57 refit by Dickson and Miller58, and Dickson for KHSO459 to calculate PCO2. Averages of salinity, temperature, pH, and carbonate system parameters are reported in Supplementary Table S1.
Physiological measurements: Brain and plasma analysis
Immediately after fish being euthanized, the brain was quickly dissected and flash frozen in a mini mortar stored in liquid nitrogen. The tissue was powdered in the mortar using a pestle stored in liquid nitrogen attached to a cordless power tool (Cryogrinder, OPS Diagnostics, New Jersey, USA). The tissue powder was then transferred to a pre-weighed cryotube, sealed, and a final weight was taken to determine tissue mass (g). Tissue homogenization and transfer to cryotubes took place in a glove box containing a CO2-free atmosphere60. A buffer containing two metabolic inhibitors, potassium fluoride (0.16 mM) and nitrilotriacetic acid (2.9 M) and adjusted to pH 7.4 with NaOH was added to the sample (250 μl/sample) as previously outlined in Pörtner 199060. This mixture was briefly vortexed, centrifuged, and immediately placed on ice. This supernatant was used for measurements of both intracellular brain pH and intracellular HCO3− (mM/kg). Contamination of extracellular fluids by this method has been deemed negligible60.
A custom built gas-tight chamber fitted with an electrode (PHC4000-8, Radiometer, France) and surrounded by an acrylic thermostated sleeve was used to measure pHi. Buffer was used to flush out the electrode chamber twice prior to processing each sample. The chamber was then flushed once with the supernatant from the homogenized tissue to clear the buffer and after which pH was recorded on a second injection of supernatant. To determine brain HCO3− (mM/kg), an aliquot of the supernatant (corresponding to 200–1200 nmol) was added to 10 mL of 50 mM NaCl for double endpoint titrations (see below). Brain PCO2 was calculated using the Henderson-Hasselbalch equation (See Supplementary Information).
Due to the inherent difficulty in sampling smaller fish and the speed required to complete brain homogenization procedures, plasma and brain samples were not taken from the same individual. Blood was drawn from the caudal vein into a heparinized syringe using a 23G hypodermic needle. Blood was briefly centrifuged for 30 sec and plasma was placed on ice for subsequent analyses.
Double endpoint titrations
Total bicarbonate and carbonate equivalents (referred to as “HCO3−” throughout) were determined in the brain supernatant and the plasma using double endpoint titrations61,62. For all samples, an aliquot was pipetted into 10 mL of a 50 mM solution of NaCl in deionized water63. Following a 15-minute period where samples were bubbled with CO2-free gas (either nitrogen or argon) to stabilize pH, initial pH was recorded (PHC 3005-8, Radiometer Analytical). Samples were titrated while being bubbled with CO2-free gas using 0.01 N HCl until a stable reading at or slightly below 3.8 was determined, bubbled with CO2-free gas for an additional 15 min, then titrated back up to the initial pH using 0.01 N NaOH. For quality control, the measured concentration of NaOH was determined to be 0.009482 N following back titrations against certified HCl standards used in this series of titrations. Additions of acid and base titrants were dispensed manually using 2 mL microburettes (GS-1200, Gilmont Instruments). Total HCO3− equivalents in the sample were determined by subtracting the moles of NaOH from the moles of HCl required to bring the sample back to the initial pH63.
Behavioural response to a chemical alarm cue
As outlined in a previous study13, the response to olfactory cues was tested by giving a fish the choice between untreated seawater and seawater containing CAC in a two-channel choice flume. As the fish used in this study were considerably larger (15.8 ± 0.5 g) than those tested by Welch et al.13 (0.12 ± 0.07 g), a larger flume was used (30 cm × 13 cm)64. Control or CAC treated water was gravity fed into either side at a constant flow rate of 450 mL/min. Validation of equal (laminar) flow rates was achieved using both a flow meter and a dye test following each water change performed every second fish. Dye tests confirmed water streams coming from each side of the flume were not mixed (see Supplementary Fig. S2).
CAC-treated seawater was made by euthanizing a donor fish of the same species with a quick blow to the head and making shallow cuts along the side of the body to mimic an injured conspecific. The fish was then rinsed with 30 mL of seawater that was collected and added to 10 L of seawater that would serve as CAC seawater in the choice system. One donor fish was used per test fish in an experimental run. Previous work has demonstrated that the behavioural response to a chemical cue is the same when presented in control and elevated CO2 water14. This finding was confirmed in a recent study showing no effect of using control vs. treatment water flume choice and escape response behavioural assays. In addition, the aforementioned study used two species previous used in CO2 behavioral experiments on Lizard Island (Amphiprion percula and Pomacentrus amboinensis)38. Fish in the choice system in this particular study were tested using control seawater.
During choice tests, fish were introduced to the center of the downstream end of the flow chamber and allowed to acclimate for 2 minutes. Following acclimation, the location of the fish was recorded every five seconds over a 2 minute recording period. During a three-minute “rest” period, the water sources were switched to eliminate any side biases and the two-minute acclimation period was repeated. The fish was gently re-centered in the flume during this time a piece of soft mesh, and the recording period was then repeated. To avoid confounding issues of handling stress, fish tested in flume trials were not used for physiological measurements outlined above.
Statistical analysis
Student t-tests were used to compare measurements from control and CO2-treated fish. Data with a priori directional predictions were assessed using a one-tailed t-test and are specifically noted in the text and figures. Data that were non-parametric were analyzed using a Mann-Whitney rank sum test. Significance was determined at P < 0.05 for all tests and all values are presented as means ± s.e.m.
Additional Information
How to cite this article: Heuer, R. M. et al. Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci. Rep. 6, 33216; doi: 10.1038/srep33216 (2016).
Supplementary Material
Acknowledgments
R.M. Heuer was supported by a National Science Foundation Graduate Research Fellowship (DGIE-0951782) and the University of Miami Koczy Fellowship and M. Grosell is a Maytag professor of Ichthyology and supported by a National Science Foundation award (IOS 1146695). This study was also funded by the ARC Centre of Excellence for Coral Reef Studies. P.L. Munday was supported by an ARC Future Fellowship and J.L. Rummer was supported by an ARC Early Career Discovery Fellowship. Special thanks goes to the staff at Lizard Island for their assistance. We wish to thank Dr. Goran Nilsson and Matthew Regan for fruitful discussions of EGABA calculations. Finally, we would like to thank Michael Jarrold for providing the image of the choice flume dye test shown in Supplementary Figure S2.
Footnotes
Author Contributions All authors designed the study and performed experiments. R.M.H., M.J.W. and M.G. analyzed the data. M.G. and P.L.M. co-supervised the project. R.M.H wrote the paper and all other authors reviewed and approved the manuscript.
References
- Heuer R. M. & Grosell M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307, R1061–R1084 (2014). [DOI] [PubMed] [Google Scholar]
- Munday P. L., Gagliano M., Donelson J. M., Dixson D. L. & Thorrold S. R. Ocean acidification does not affect the early life history development of a tropical marine fish. Marine Ecology-Progress Series 423, 211–221, 10.3354/Meps08990 (2011). [DOI] [Google Scholar]
- Franke A. & Clemmesen C. Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences 8, 3697–3707 (2011). [Google Scholar]
- Strobel A., Graeve M., Poertner H. O. & Mark F. C. Mitochondrial acclimation capacities to ocean warming and acidification are limited in the Antarctic Nototheniid fish, Notothenia rossii and Lepidonotothen squamifrons. PloS one 8, e68865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A. et al. Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and PCO2. Frontiers in Zoology 9, 10.1186/1742-9994-9-28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummer J. L. et al. Elevated CO2 enhances aerobic scope of a coral reef fish. Conservation Physiology 1, 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley D. M. Jr. et al. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683 (2009). [DOI] [PubMed] [Google Scholar]
- Bignami S., Enochs I. C., Manzello D. P., Sponaugle S. & Cowen R. K. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proceedings of the National Academy of Sciences of the United States of America 110, 7366–7370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. M., Watson S. A., McCormick M. I. & Munday P. L. Increased CO2 stimulates reproduction in a coral reef fish. Global Change Biology 19, 3037–3045, 10.1111/gcb.12259 (2013). [DOI] [PubMed] [Google Scholar]
- Welch M. J. & Munday P. L. Contrasting effects of ocean acidification on reproduction in reef fishes. Coral Reefs, 1–9 (2015). [Google Scholar]
- Heuer R. M., Esbaugh A. J. & Grosell M. Ocean acidification leads to counterproductive intestinal base loss in the gulf toadfish (Opsanus beta). Physiological and Biochemical Zoology 85, 450–459 (2012). [DOI] [PubMed] [Google Scholar]
- Esbaugh A. J., Heuer R. & Grosell M. Impacts of ocean acidification on respiratory gas exchange and acid-base balance in a marine teleost, Opsanus beta. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 182, 921–934 (2012). [DOI] [PubMed] [Google Scholar]
- Welch M. J., Watson S.-A., Welsh J. Q., McCormick M. I. & Munday P. L. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nature Climate Change 4, 1086–1089 (2014). [Google Scholar]
- Munday P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proceedings of the National Academy of Sciences 107, 12930–12934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday P. L. et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences 106, 1848–1852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. D. et al. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biology letters 7, 917–920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-S., Marshall N. J., Watson S.-A., Munday P. L. & Nilsson G. E. Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. The Journal of Experimental Biology 217, 323–326 (2014). [DOI] [PubMed] [Google Scholar]
- Ferrari M. C. et al. Effects of ocean acidification on visual risk assessment in coral reef fishes. Functional Ecology 26, 553–558 (2012). [Google Scholar]
- Domenici P., Allan B., McCormick M. I. & Munday P. L. Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biology Letters 8, 78–81, 10.1098/rsbl.2011.0591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutfelt F., Bresolin de Souza K., Vuylsteke A. & Sturve J. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PloS one 8, e65825, 10.1371/journal.pone.0065825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F., Jutfelt F. & Nilsson G. E. Altered neurotransmitter function in CO2-exposed stickleback (Gasterosteus aculeatus): a temperate model species for ocean acidification research. Conservation Physiology 3, cov018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers D. P. et al. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Global change biology 20, 515–522 (2013). [DOI] [PubMed] [Google Scholar]
- Ferrari M. C. et al. Effects of ocean acidification on learning in coral reef fishes. PloS one 7, e31478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday P. L., Cheal A. J., Dixson D. L., Rummer J. L. & Fabricius K. E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nature Climate Change 4, 487–492 (2014). [Google Scholar]
- Caldeira K. & Wickett M. E. Anthropogenic carbon and ocean pH. Nature 425, 365–365, 10.1038/425365a (2003). [DOI] [PubMed] [Google Scholar]
- Munday P. L., McCormick M. I. & Nilsson G. E. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? The Journal of experimental biology 215, 3865–3873 (2012). [DOI] [PubMed] [Google Scholar]
- Claiborne J. B., Edwards S. L. & Morrison-Shetlar A. I. Acid-base regulation in fishes: Cellular and molecular mechanisms. Journal of Experimental Zoology 293, 302–319 (2002). [DOI] [PubMed] [Google Scholar]
- Ishimatsu A., Kikkawa T., Hayashi M., Lee K. S. & Kita J. Effects of CO2 on marine fish: larvae and adults. Journal of Oceanography 60, 731–741 (2004). [Google Scholar]
- Toews D. P., Holeton G. F. & Heisler N. Regulation of the acid-base status during environmental hypercapnia in the marine teleost fish Conger conger. J Exp Biol 107, 9–20 (1983). [DOI] [PubMed] [Google Scholar]
- Brauner C. J. & Baker D. W. Patterns of Acid-Base Regulation during Exposure to Hypercarbia in Fishes. 43–63 (Springer, 2009). [Google Scholar]
- Larsen B. K. & Jensen F. Influence of ionic composition on acid-base regulation in rainbow trout (Oncorhynchus mykiss) exposed to environmental hypercapnia. Fish Physiology and Biochemistry 16, 157–170 (1997). [Google Scholar]
- Green L. & Jutfelt F. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biology letters 10, 20140538 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbaugh A., Ern R., Nordi W. & Johnson A. Respiratory plasticity is insufficient to alleviate blood acid-base disturbances after acclimation to ocean acidification in the estuarine red drum, Sciaenops ocellatus. Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology (2015). [DOI] [PubMed] [Google Scholar]
- Nilsson G. E. et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Climate Change 2, 201–204, doi: http://www.nature.com/nclimate/journal/v2/n3/abs/nclimate1352.html#supplementary-information (2012). [Google Scholar]
- Hamilton T. J., Holcombe A. & Tresguerres M. CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABAA receptor functioning. Proceedings of the Royal Society B: Biological Sciences 281, 20132509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou M. et al. Responses of pink salmon to CO2-induced aquatic acidification. Nature Climate Change (2015). [Google Scholar]
- Regan M. D. et al. Ambient CO2, fish behaviour and altered GABAergic neurotransmission: exploring the mechanism of CO2-altered behaviour by taking a hypercapnia dweller down to low CO2 levels. J Exp Biol 219, 109–118 (2016). [DOI] [PubMed] [Google Scholar]
- Munday P. et al. Effects of elevated CO2 on predator avoidance behaviour by reef fishes is not altered by experimental test water. bioRxiv 050062, doi: http://dx.doi.org/10.1101/050062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S.-A. et al. Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proceedings of the Royal Society of London B: Biological Sciences 281, 20132377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier R. G., Heming T. A. & Iwama G. K. Appendix: Physicochemical parameters for use in fish respiratory physiology. Fish Physiology. Volume X Gills. Part A: Anatomy, Gas transfer, and acid-base regulation (1984). [Google Scholar]
- Baker D. W. et al. Complete intracellular pH protection during extracellular pH depression is associated with hypercarbia tolerance in white sturgeon, Acipenser transmontanus. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 296, R1868–R1880 (2009). [DOI] [PubMed] [Google Scholar]
- Wood C. M. & Lemoigne J. Intracellular acid-base responses to environmental hyperoxia and normoxic recovery in rainbow-trout. Respiration Physiology 86, 91–113 (1991). [DOI] [PubMed] [Google Scholar]
- Wood C., Turner J., Munger R. & Graham M. Control of ventilation in the hypercapnic skate Raja ocellata: II. Cerebrospinal fluid and intracellular pH in the brain and other tissues. Respiration physiology 80, 279–297 (1990). [DOI] [PubMed] [Google Scholar]
- Heinrich D. D. et al. A product of its environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conservation Physiology 2, cou047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. F. et al. Acid-base regulation in the plainfin midshipman (Porichthys notatus): an aglomerular marine teleost. Journal of Comparative Physiology 180, 1213–1225 (2010). [DOI] [PubMed] [Google Scholar]
- Ern R. & Esbaugh A. J. Hyperventilation and blood acid–base balance in hypercapnia exposed red drum (Sciaenops ocellatus). Journal of Comparative Physiology B, 1–14 (2016). [DOI] [PubMed] [Google Scholar]
- Caldwell S., Rummer J. L. & Brauner C. J. Blood sampling techniques and storage duration: effects on the presence and magnitude of the red blood cell β-adrenergic response in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 144, 188–195 (2006). [DOI] [PubMed] [Google Scholar]
- Baker D. W., Sardella B., Rummer J. L., Sackville M. & Brauner C. J. Hagfish: Champions of CO2 tolerance question the origins of vertebrate gill function. Scientific reports 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M. & Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Progress in Brain Research 160, 59–87 (2007). [DOI] [PubMed] [Google Scholar]
- Delpire E. & Staley K. J. Novel determinants of the neuronal Cl− concentration. The Journal of Physiology 592, 4099–4114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N. & Grover L. The mechanism of biphasic GABA responses. Science 269, 928–929 (1995). [DOI] [PubMed] [Google Scholar]
- Sundin J. & Jutfelt F. 9–28 d of exposure to elevated pCO2 reduces avoidance of predator odour but had no effect on behavioural lateralization or swimming activity in a temperate wrasse (Ctenolabrus rupestris). ICES Journal of Marine Science: Journal du Conseil, fsv101 (2015). [Google Scholar]
- Jutfelt F. & Hedgärde M. Juvenile Atlantic cod behavior appears robust to near-future CO2 levels. Frontiers in Zoology 12, 11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. P. Steroid modulation of GABA A receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology 52, 1439–1453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Belelli D., Peden D. R., Vardy A. W. & Peters J. A. Neurosteroid modulation of GABA-A receptors. Progress in Neurobiology 71, 67–80 (2003). [DOI] [PubMed] [Google Scholar]
- Hari P. et al. High-frequency measurements of productivity of planktonic algae using rugged nondispersive infrared carbon dioxide probes. Limnol Oceanogr Methods 6, 347–354 (2008). [Google Scholar]
- Mehrbach C., Culberson C. H., Hawley J. E. & Pytkowicz R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- Dickson A. G. & Millero F. J. A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep-Sea Research Part a-Oceanographic Research Papers 34, 1733–1743 (1987). [Google Scholar]
- Pierrot D., Lewis E. & Wallace D. M. S. Excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee (2006). [Google Scholar]
- Pörtner H.-O. Determination of intracellular buffer values after metabolic inhibition by fluoride and nitrilotriacetic acid. Respiration physiology 81, 275–288 (1990). [DOI] [PubMed] [Google Scholar]
- Genz J., Taylor J. R. & Grosell M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid-base balance in Opsanus beta. J Exp Biol 211, 2327–2335 (2008). [DOI] [PubMed] [Google Scholar]
- Brix K. V., Wood C. M. & Grosell M. Measuring titratable alkalinity by single versus double endpoint titration: An evaluation in two cyprinodont species and implications for characterizing net H+ flux in aquatic organisms. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 164, 221–228 (2013). [DOI] [PubMed] [Google Scholar]
- Grosell M., De Boeck G., Johannsson O. & Wood C. M. The effects of silver on intestinal ion and acid-base regulation in the marine teleost fish, Papophrys vetulus. Comparative Biochemistry and Physiology 124, 259–270 (1999). [DOI] [PubMed] [Google Scholar]
- Schunter C. et al. Molecular signatures of transgenerational response to ocean acidification in a reef fish. Nature Climate Change In Press (2016). [Google Scholar]
- Grosell M. Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiologica 202, 421–434, 10.1111/j.1748-1716.2010.02241.x (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.