Fig. 6.
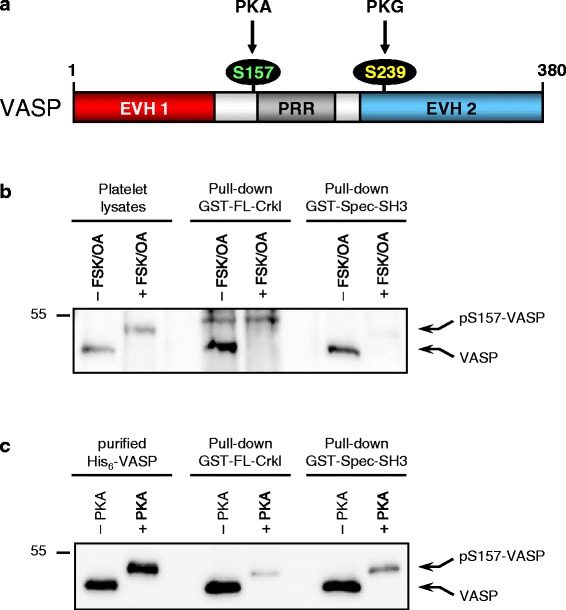
PKA-mediated VASP phosphorylation abrogates Crkl-VASP interaction. a Domain organization and phosphorylation sites of human VASP (380 aa); EVH1/2 Ena/VASP homology 1/2 domain; PRR, proline-rich region. VASP is a prominent substrate of cyclic nucleotide-dependent serine/threonine kinases. Human VASP is preferentially phosphorylated by PKA at serine 157 (S157, green) and by PKG at serine 239 (S239, yellow). Please note that S157 is located in close proximity to the PRR, which is important for SH3-domain mediated interactions. b Human platelets were stimulated or not with a combination of FSK and OA to induce PKA-mediated VASP phosphorylation at Ser157 (pS157-VASP). Equal amounts of lysates from unstimulated or stimulated platelets were incubated with immobilized GST-FL-Crkl or GST-Spec-SH3 and interaction of VASP with the GST-fusion proteins was determined as described in the legend to Fig. 5c and d. c. Equal amounts of purified His6-tagged VASP were S157-phosphorylated in vitro by PKA (+PKA) or left untreated (−PKA) and pulled-down with immobilized GST-FL-Crkl or GST-Spec-SH3. Interaction of VASP wih the GST-fusion proteins was determined as described in the legend to Fig. 5c and d. Please note that VASP Ser157-phosphorylation (but not S239-phosphorylation) induces a shift in the apparent molecular weight from 46 to 50 kDa
