Figure 4.
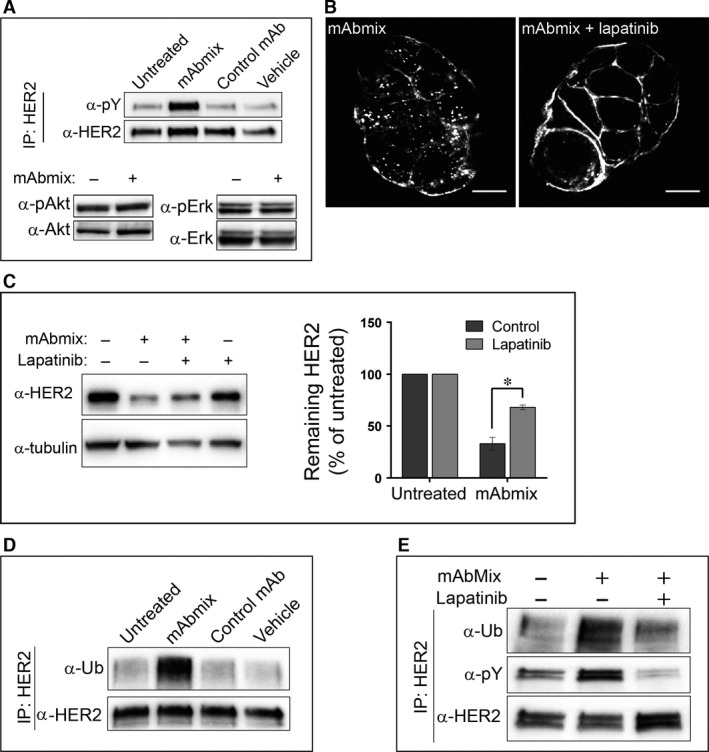
Antibody‐induced ubiquitination, internalization and degradation of HER2 depend on HER2 kinase activity. (A) OE19 cells were incubated in MEM only (Untreated) or in MEM with the mAb mixture, the negative control mAb or the dilution buffer for antibodies (Vehicle) for 1 hr. HER2 was immunoprecipitated and the immunoprecipitated material was analysed by immunoblotting using an antibody to HER2 (clone Z4881) before the membrane was stripped and re‐probed with an antibody to phosphorylated tyrosine (pY). Whole‐cell lysates were immunoblotted using antibodies to phosphorylated Akt (pAkt), Akt1, phosphorylated Erk1/2 (pErk) and Erk1. One representative experiment of three is shown. (B) OE19 cells were pre‐incubated in MEM with or without lapatinib (1 μM) for 1 hr, followed by incubation in MEM containing the mAb mixture for 4 hrs in absence or presence of lapatinib, before fixation, permeabilization and immunostaining for human IgG. Micrographs representative for three experiments are shown, scale bars: 10 μm. (C) OE19 cells were incubated in growth medium with the mAb mixture in absence or presence of lapatinib (1 μM) for 24 hrs, before lysis and immunoblotting using an antibody to HER2 (clone Z4881). Tubulin was used as loading control. One representative experiment of three is shown. Net luminescence of bands corresponding to HER2 was quantified and normalized to the loading control, and the average values of at least three independent experiments ±S.D. were plotted as percentage of HER2 in untreated cells. (D) OE19 cells were incubated in MEM only (Untreated) or in MEM with the mAb mixture, the negative control mAb or the dilution buffer for antibodies (Vehicle) for 1 hr before immunoprecipitation of HER2. The immunoprecipitates were subjected to immunoblotting using antibodies to ubiquitin (Ub). The membrane was stripped and re‐probed with an antibody to HER2 (clone Z4881). One representative experiment of three is shown. (E) OE19 cells were incubated in MEM only or pre‐incubated in MEM with or without lapatinib (1 μM) for 1 hr, followed by incubation in MEM with the mAb mixture with or without lapatinib for 1 hr. HER2 was immunoprecipitated and the immunoprecipitates were subjected to immunoblotting using antibodies to ubiquitin (Ub). The membrane was stripped and re‐probed with antibodies to phosphorylated tyrosine (pY) and to HER2 (clone Z4881). One representative experiment of three is shown.
