Figure 5.
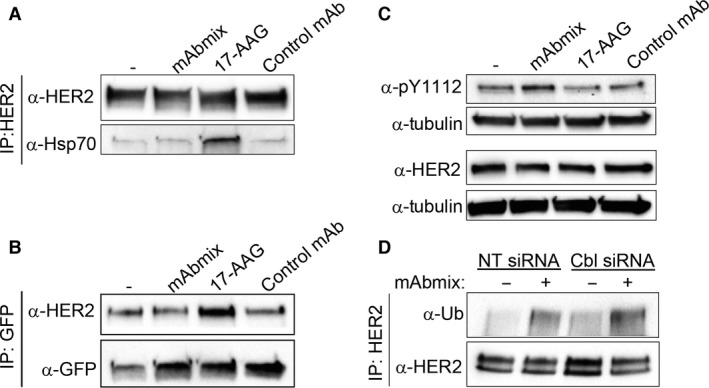
Antibody‐induced ubiquitination of HER2 is independent of both Hsp70 recruitment and the ubiquitin ligase Cbl. (A) OE19 cells were incubated in MEM only or in MEM with the mAb mixture, 17‐AAG (3 μM) or the negative control mAb for 1 hr, before co‐immunoprecipitation analysis using an antibody to HER2 (29D8). The immunoprecipitates were subjected to immunoblotting using antibodies to HER2 (clone 42) and to Hsp70. One representative experiment of three is shown. (B) OE‐19 cells were transiently transfected with plasmid‐encoding GFP‐tagged CHIP. Cells were incubated in MEM only or in MEM with the mAb mixture, 17‐AAG (3 μM) or the negative control mAb for 1 hr, before co‐immunoprecipitation analysis using GFP‐Trap®. The immunoprecipitated proteins and total cell lysates were analysed by immunoblotting using antibodies to HER2 (clone Z4881) and GFP. (C) OE19 cells were incubated in MEM only or in MEM with the mAb mixture, 17‐AAG (3 μM) or the negative control antibody for 1 hr. Cell lysates were subjected to immunoblotting using antibodies to phosphorylated tyrosine 1112 in HER2 (pY1112), HER2 (clone 42) and to tubulin (loading control). One representative experiment of three is shown. (D) Cbl‐b and c‐Cbl were knocked down in OE19 cells using siRNA as described in Materials and Methods. Non‐targeting (NT) siRNA was used as control. Cells were incubated in MEM with or without the mAb mixture for 1 hr, before HER2 was immunoprecipitated under denaturing conditions. The immunoprecipitates were subjected to immunoblotting using antibodies to ubiquitin (Ub). The membrane was stripped and re‐probed with antibody to HER2 (clone Z4881). One representative experiment of three is shown.
