Abstract
Aging induces physical deterioration, loss of the blood brain barrier, neuronal loss-induced mental and neurodegenerative diseases. Hypotalamus-hypophysis-gonad axis aging precedes symptoms of menopause or andropause and is a major determinant of sensory and cognitive integrated function. Sexual steroids support important functions, exert pleiotropic effects in different sensory cells, promote regeneration, plasticity and health of the nervous system. Their diminution is associated with impaired cognitive and mental health and increased risk of neurodegenerative diseases. Then, restoring neuroendocrine axes during aging can be key to enhance brain health through neuroprotection and neuroregeneration, depending on the modulation of plasticity mechanisms. Estrogen-dependent transient receptor potential cation channel, subfamily V, member 1 (TRPV1) expression induces neuroprotection, neurogenesis and regeneration on damaged tissues. Agonists of TRPV1 can modulate neuroprotection and repair of sensitive neurons, while modulators as other cognitive enhancers may improve the survival rate, differentiation and integration of neural stem cell progenitors in functional neural network. Menopause constitutes a relevant clinical model of steroidal production decline associated with progressive cognitive and mental impairment, which allows exploring the effects of hormone therapy in health outcomes such as dysfunction of CNS. Simulating the administration of hormone therapy to virtual menopausal individuals allows assessing its hypothetical impact and sensitivity to conditions that modify the effectiveness and efficiency.
Keywords: TRPV1, estrogen, hormonal replacent, neuroprotection, aging
Introduction
Reproductive aging even precedes the clinical manifestations of menopause or andropause and is a major determinant in sexual behavior and maintenance of optimal cognitive functions. This neurodegenerative environment is characterized by neuronal loss, physical deterioration, loss of the blood-brain barrier and systemic inflammation. Thus, the control of neuroendocrine changes (Devoto et al., 2012) in the aging process may be crucial to maintain sexual behavior and improve brain function through neuroprotection or neurogenesis. Estrogen and its metabolites are important paracrine modulators of the function of hypophysis (García-Barrado et al., 2016), corpus luteum (Henríquez et al., 2016) and hippocampus (Farinetti et al., 2015), being determinant on ovarian and brain performance during aging process. Estrogen replacement therapy can decrease risk of memory disturbances in menopausal women (Rettberg et al., 2016b). Indeed, brain synthesis of steroids is able to modulate several mechanisms related to neurogenesis, neural plasticity and memory functions. Peripheral steroid hormones also have important regulatory effects on CNS. In animal models, ovarian cycle modulates abundance and location of neurotrophic factor in hippocampal neurons. Estradiol modulates neuroplasticity showing sex-specific differences in synaptogenesis in adult hippocampus (Brandt et al., 2013). In turn, the hippocampus is able to synthesize estrogens that are implied in learning processes and spatial and contextual memory. Either locally produced or exogenous steroids may provide neuroprotective and neuroregenerative effects. Specifically, exogenous estradiol increases the formation of dendritic spines in hippocampus and medial prefrontal cortex of ovariectomized female mice (Tuscher et al., 2016) in support of possible beneficial effects on memory. These findings have added new information on use of estrogen receptor ligands for improving cognitive performance in postmenopausal women. There is ample evidence indicating that estrogens and their metabolites are able to promote regeneration, plasticity and health of the nervous system, while its decrease or absence is associated with impaired physical and mental health besides increased risk of cardiovascular and neurodegenerative diseases (Bean et al., 2015). The efficacy of estrogen therapy in neurodegenerative processes has nevertheless been controversial given the heterogeneity of clinical results besides some discrepancies between experimental and clinical studies. For instance, hormone treatment has been reported to have beneficial effects on the risk of Alzheimer's disease in menopausal women, whereas other reports question its overall benefits. These discrepancies may be due to genetic and hormonal differences, time at onset of neurodegenerative disorders and time at which hormone treatment is started, the type and possibly the scheduling of hormone treatment (Villa et al., 2016). Detrimental effects of hormone treatment on heart attack, stroke or deep vein thrombosis may be due to pro-thrombotic actions of estrogens in individuals with acute disruption of atherosclerotic plaques (Liu and Yang, 2013). The main obstacle to study the effects and design suitable hormone therapies is the fact that estrogens do not seem to play a primary role as promoters of the neurodegenerative program but participate only in the complex combination of events that trigger neurodegeneration. The success of estrogen therapies would thus depend on a careful consideration of not only the pathway being targeted by the treatment but also the sources of variability of effects, such as the nature of the disease process, or the amplification of effects mediated by modulators as TRP (transient receptor potential) V1 channel. The molecular identity of the estrogen-dependent pathway responsible to activate neuroprotection is unknown. We propose that TRP ion channel family, and specifically transient receptor potential cation channel, subfamily V, member 1 (TRPV1), are able to act like ionotropic receptors of steroids offering a novel pharmacological target in neuroprotection and neuroregeneration. In this commentary we focus on the TRPV1 effects related to promotion of cell protection and self-repair, which could be particularly important in phenomena underlying neurodegenerative diseases and central nervous system repair, as well as in cell therapy development.
Estrogens-induced Neuroprotection and Neuroregeneration
Estrogens comprise a group of structurally related molecules among which 17β-estradiol is the most predominant and active. Estrogens are cholesterol derived-molecules produced mainly by gonads, but also adrenal gland and hippocampus, which express the enzyme aromatase, are able to produce estrogens from androgens. Estrogens perform important physiological functions and exert pleiotropic effects in different cell types and organs including immune, bone, gastrointestinal, respiratory, reproductive, cardiovascular and nervous system. In brain, estrogens exert several effects as embryonic neurogenesis, excitatory synapse formation, neuroprotective and cognitive-enhancing effects and memory. These effects are elicited by multiple pathways involving regulation of neurotransmitter release, increase in the number of dendritic spines, regulation of calcium homeostasis and improvement of mitochondrial function (Zhang et al., 2016).
Classically, estrogens have been thought to exert their effects via a genomic mechanism involving binding of estrogen to nuclear receptors and subsequent regulation of specific gene transcription. The genomic action typically takes several hours for the effect to be manifest due to either transcription and translation times of estrogen-regulated genes. In physiological conditions estrogens can regulate ubiquitous cellular processes but also dependent or independent on classical pathway. The regulatory effects of estrogens have raised great interest due to evidence that estrogens may delay onset or reduce the severity of several neurological disorders, as well as ischemia-reperfusion brain damage (Siddiqui et al., 2016).
The effect of estrogens in CNS could be related with two important functions: 1) promoting cell proliferation and differentiation and 2) neuroprotection against oxidative injuries. Recent data have highlighted the role of estrogens in neurogenic activity in mammals and other vertebrates. Ovariectomized female rats exhibit a decrease in neurogenesis in dentate gyrus, whichcan be reversed by 17β-estradiol. Similar effects have been detected in the subventricular zone of the forebrain and the subgranular zone of the dentate gyrus of the hippocampus. It has been reported that 17β-estradiol increases the ratio of neurons toglia cells in neural stem cells in vitro and that it could modulate the differentiation of embryonic stem cells inducing neuronal phenotype (Murashov et al., 2004). Treatment with estradiol induces important effects on synapse formation such as increased neurite outgrowth and dendrite spine formation (Vierk et al., 2014; Gervais et al., 2015).
Additionally, 17β-estradiol modulates neuron survival and participates in brain repair. Estradiol powerfully protects the brain against damage caused by mechanical or chemical injury by activating multiple mechanisms. The proposed cellular mechanisms to prevent cell death in models as stroke, deprivation, or oxidative environment, involve mitochondrial membrane potential improvement, prevention of ATP decay and reduction of ROS generation, via increased expression of anti-apoptotic proteins like bcl-2 and inhibition of pro-apoptotic proteins like Bim and Bad. Aromatase knock-out adult female rats exhibit exaggerated spontaneous apoptosis in neurons of the frontal cortex compared to wild type animals strengthening the anti-apoptotic role of estrogens.
A large body of evidence has currently emerged showing rapid effects of high-concentration estrogens that appear to involve membrane-associated signaling complexes capable of responding within seconds or minutes. The kinetics of such responses are inconsistent with a mechanism requiring gene transcription and may be independent of estrogen receptors. For instance, 17β-estradiol conjugated to BSA has been shown to be an impermeable plasma membrane compound and has been used to study the role of membrane ER in producing the non-genomic effects of estradiol. These rapid non-genomic actions of estrogens involve mainly modulation of ion channels conduction and permeation and transient increase of intracellular Ca2+, which are related with signaling pathways that are critical for plasticity, cognition, neuroprotection and neurogenesis.
The diversity of estrogens mechanisms of action amplifies the regulatory possibilities of cellular stress response and regeneration. It is likely that estrogens are able to induce both cell responses, non-genomic and genomic, controlling cellular reactions to acute stress that are critical to maintain the viability and long-term effects that promote cell survival and regeneration. Yet, the still unclear mechanisms of rapid action of estrogens are possibly key to understand how estrogens exert their purported beneficial effects.
TRP as Molecular Target for Estrogen-induced Neural Function
Steroids exert pleiotropic functions not only through nuclear receptor, but also via a direct interaction with membrane receptors. 17β-estradiol binds to the β subunit of the Maxi-K potassium channel and activates it in vascular smooth muscle and in neuron-derived cell line, 17β-estradiol also binds directly to β subunit of L-type calcium channel enhancing their activity.
The polymodal transient receptor potential ion channels (TRP) family comprises a broad variety of non-selective cationic channels that are able to integrate multiple physicochemical stimuli including voltage, temperature, osmolality and several hydrophobic ligands such a vanilloids and phospholipids (Diaz-Franulic et al., 2016) have been proposed as potential targets of steroids (Nilius and Voets, 2008). Regarding TRPV activity, steroids regulate transepithelial Ca2+ transport, contributing to whole body Ca2+ homeostasis. Besides these rapid effects, 17β-estradiol increases the expression of TRPV1 in uterine cervical afferent neurons as well as in dorsal root ganglion cells through mechanisms that are dependent on ERβ and ERβ, suggesting reproductive cycle control of nociception in primary afferent neurons (Cho and Chaban, 2012).
Steroids Modulation of TRPV1-A Curious Fact
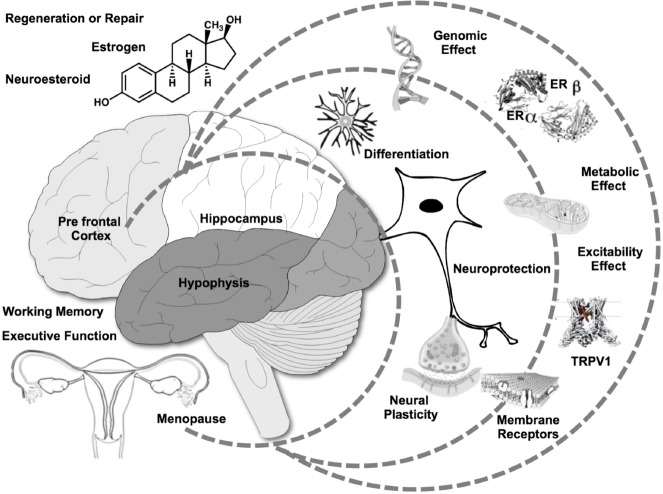
Modification of TRPV1 hydrophobic environment can alter biophysical properties and function of the channel. It seems that different molecules could work cooperatively in channel activation, and some of them like PIP2, cholesterol or capsaicin bind to a promiscuous hydrophobic pocket within the channel (Gao et al., 2016). While almost all steroids tested in TRPV1 inhibit capsaicin-induced currents in rat dorsal root ganglion neurons, few reports indicate that only 17β-estradiol could enhance these currents and the expression of the channel at least in dorsal root ganglion neurons, and little is known about direct TRPV1 modulation by estrogens. Since estrogens have been involved in neural protection and regeneration in several organs and predominantly in the brain, understanding their role on TRPV1 activity is of particular interest to characterize these processes. Several data suggest that estrogen metabolites and precursors like cholesterol and testosterone can inhibit TRPV1 activity. It is likely that estrogens and its precursors occupy competitively the same hydrophobic pocket but only the aromatic steroid 17β-estradiol is able to activate the channel, what could partly explain its neurogenic or neuroprotective functions. Under this hypothesis, 17β-estradiol might enhance endogenous neuroprotective mechanisms and foster favorable environment to stem cells grafts survival. The control of this mechanism could generate molecular strategies to decrease oxidative stress-induced neural progenitor death (Ramirez-Barrantes et al., 2016) (Figure 1).
Figure 1.
Model of the effect of estrogens on cognitive performance improvement.
Molecular, cellular and tissue hypotheses integration for multilevel mechanisms of action to be tested in clinical trials. Physiological and pharmacological relationships at different levels of complexity lead into neuroprotective effects that are clinically relevant. TRPV1 channel offers a novel pharmacological target that may modulate the actions of estrogens with significant implications on clinical efficacy. ER: Estrogen receptor; TRPV1: transient receptor potential cation channel, subfamily V, member 1.
Estrogens Improve Cognitive Function: A Health Point of View
Certainly, stem cell transplantation is the most promising technique for the treatment of nervous system damage. Specifically, regenerative perspective should include the cellular fate of damaged cells or implanted cells through steroid-dependent stimulation (Labombarda and Garcia-Ovejero, 2014), growth factors, cytokines and neurotransmitters (Singh et al., 2012; Datto et al., 2015). Although the beneficial effects of stem cell transplantation in experimental models have been widely documented, the development of a massive technology depends on the application and reproducibility of results in clinical environment.
A neurodegenerative environment induces neuronal loss, physical deterioration, loss of the blood brain barrier and systemic inflammation. There is ample evidence supporting promotion of regeneration, plasticity and health of the nervous system by steroids and their metabolites (Labombarda and Garcia-Ovejero, 2014), while its diminution or absence is associated with impaired physical and mental health and increased risk of cardiovascular disease and neurodegenerative diseases. The effectiveness of a pro-regenerative therapeutic agent for systemic use can be tested in clinical trials. Menopause constitutes a relevant model of estrogens production decline associated with progressive cognitive impairment related to ageing (Rettberg et al., 2016a), which allows exploring the role of TRPV1 as a mediator of the therapeutic effects of exogenous steroids. Exploring these hypotheses in the clinical arena may help proposing personalized strategies of hormone replacement therapy focused on sexual and cognitive health.
Footnotes
Funding: Our work has been supported by grants Fondecyt1140693 - 11100047- 11110399 - ECOS 12029.
Conflicts of interest: None declared.
References
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, Golde TE, Foster TC. Re-opening the critical window for estrogen therapy. J Neurosci. 2015;35:16077–16093. doi: 10.1523/JNEUROSCI.1890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Vierk R, Rune GM. Sexual dimorphism in estrogen-induced synaptogenesis in the adult hippocampus. Int J Dev Biol. 2013;57:351–356. doi: 10.1387/ijdb.120217gr. [DOI] [PubMed] [Google Scholar]
- Cho T, Chaban VV. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-α and estrogen receptor-β knockout mice. Neuroreport. 2012;23:530–534. doi: 10.1097/WNR.0b013e328353fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto JP, Yang J, Dietrich WD, Pearse DD. Does being female provide a neuroprotective advantage following spinal cord injury? Neural Regen Res. 2015;10:1533–1536. doi: 10.4103/1673-5374.165213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto L, Palomino A, Céspedes P, Kohen P. Neuroendocrinology and ovarian aging. Gynecol Endocrinol 28 Suppl. 2012;1:14–17. doi: 10.3109/09513590.2012.651927. [DOI] [PubMed] [Google Scholar]
- Diaz-Franulic I, Poblete H, Miño-Galaz G, González C, Latorre R. Allosterism and Structure in Thermally Activated Transient Receptor Potential Channels. Annu Rev Biophys. 2016;45:371–398. doi: 10.1146/annurev-biophys-062215-011034. [DOI] [PubMed] [Google Scholar]
- Farinetti A, Tomasi S, Foglio B, Ferraris A, Ponti G, Gotti S, Peretto P, Panzica GC. Testosterone and estradiol differentially affect cell proliferation in the subventricular zone of young adult gonadectomized male and female rats. Neuroscience. 2015;286:162–170. doi: 10.1016/j.neuroscience.2014.11.050. [DOI] [PubMed] [Google Scholar]
- Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Barrado MJ, Blanco EJ, Catalano-Iniesta L, Sanchez-Robledo V, Iglesias-Osma MC, Carretero-Hernández M, Rodríguez-Cobos J, Burks DJ, Carretero J. Relevance of pituitary aromatase and estradiol on the maintenance of the population of prolactin-positive cells in male mice. Steroids. 2016;111:121–126. doi: 10.1016/j.steroids.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Mumby DG, Brake WG. Attenuation of dendritic spine density in the perirhinal cortex following 17β-estradiol replacement in the rat. Hippocampus. 2015;25:1212–1216. doi: 10.1002/hipo.22479. [DOI] [PubMed] [Google Scholar]
- Henríquez S, Kohen P, Xu X, Veenstra TD, Muñoz A, Palomino WA, Strauss JF 3r5, Devoto L. Estrogen metabolites in human corpus luteum physiology: differential effects on angiogenic activity. Fertil Steril. 2016;106:230–237.e1. doi: 10.1016/j.fertnstert.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Garcia-Ovejero D. Give progesterone a chance. Neural Regen Res. 2014;9:1422–1424. doi: 10.4103/1673-5374.139456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang SH. Window of opportunity: Estrogen as a treatment for ischemic stroke. Brain Res. 2013;1514:83–90. doi: 10.1016/j.brainres.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Pak ES, Hendricks WA, Tatko LM. 17β-Estradiol enhances neuronal differentiation of mouse embryonic stem cells. FEBS Lett. 2004;569:165–168. doi: 10.1016/j.febslet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. A TRP channel-steroid marriage. Nat Cell Biol. 2008;10:3–4. doi: 10.1038/ncb1208-1383. [DOI] [PubMed] [Google Scholar]
- Ramirez-Barrantes R, Cordova C, Poblete H, Muñoz P, Marchant I, Wianny F, Olivero P. Perspectives of TRPV1 function on the neurogenesis and neural plasticity. Neural Plast 2016. 2016 doi: 10.1155/2016/1568145. 1568145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Dang H, Hodis HN, Henderson VW, John JAS, Mack WJ, Diaz Brinton R. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: Potential for detecting an at-Alzheimer's risk metabolic phenotype. Neurobiol Aging. 2016a;40:155–163. doi: 10.1016/j.neurobiolaging.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Dang H, Hodis HN, Henderson VW, St. John JA, Mack WJ, Brinton RD. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: Potential for detecting an at-Alzheimer's risk metabolic phenotype. Neurobiol Aging. 2016b;40:155–163. doi: 10.1016/j.neurobiolaging.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AN, Siddiqui N, Khan RA, Kalam A, Jabir NR, Kamal MA, Firoz CK, Tabrez S. Neuroprotective role of steroidal sex hormones: An overview. CNS Neurosci Ther. 2016;22:342–350. doi: 10.1111/cns.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PL, Agarwal N, Barrese JC, Heary RF. Current therapeutic strategies for inflammation following traumatic spinal cord injury. Neural Regen Res. 2012;7:1812–1821. doi: 10.3969/j.issn.1673-5374.2012.23.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36:1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience. 2014;274:24–32. doi: 10.1016/j.neuroscience.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation and neurodegeneration. Endocr Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Trushin S, Christensen TA, Bachmeier B V, Gateno B, Schroeder A, Yao J, Itoh K, Sesaki H, Poon WW, Gylys KH, Patterson ER, Parisi JE, Diaz Brinton R, Salisbury JL, Trushina E. Altered brain energetics induces mitochondrial fission arrest in Alzheimer's Disease. Sci Rep. 2016;6:18725. doi: 10.1038/srep18725. [DOI] [PMC free article] [PubMed] [Google Scholar]