Figure 2.
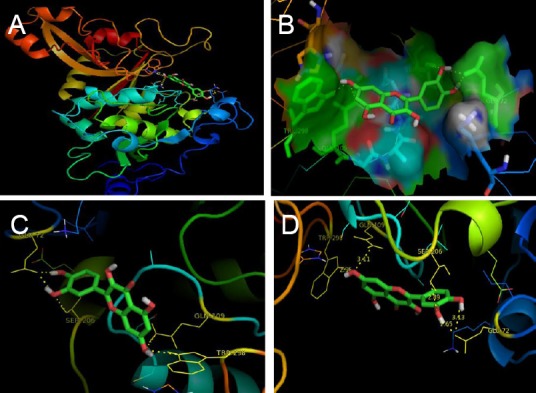
Interaction of quercetin with active site residues of μ-calpain.
(A) Position of quercetin in binding pocket (having residues Glu 72, Ser 206, Gln 109 and Trp 298) of μ-calpain at dII domain for its inhibition. (B) Interaction of quercetin with catalytic triad of calpain (green color shows the interacting residue of calpain with quercetin (rainbow color). (C) Closer view of catalytic site (having residues Glu 72, Ser 206, Gln 109 and Trp 298) occupied by quercetin in μ-calpain. (D) Closer view of quercetin interacting with the side chain of active site residue (yellow dashes indicate hydrogen bonding with active site residues).
