Figure 5.
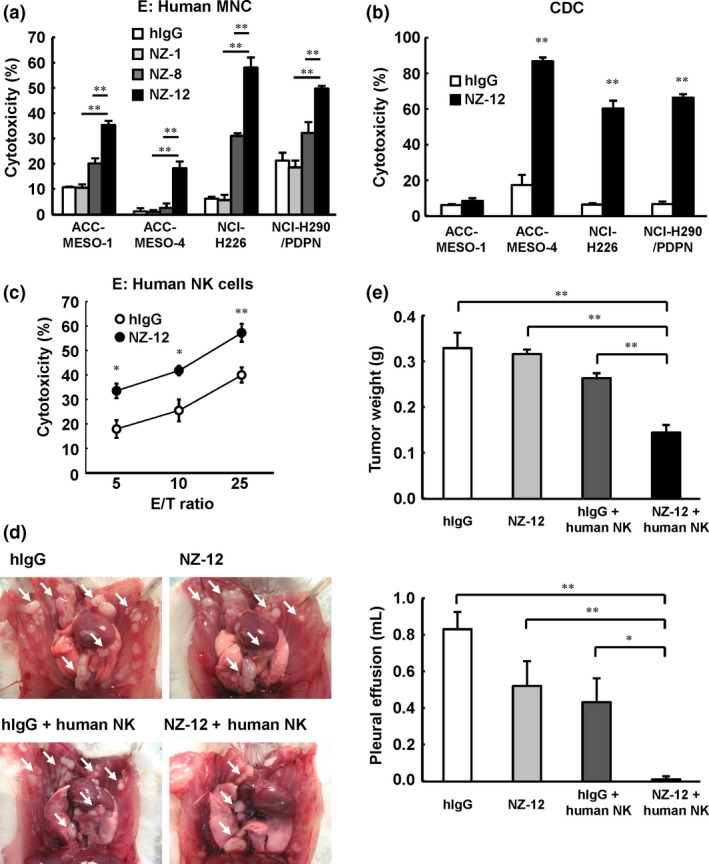
Antitumor effects of novel rat–human chimeric anti‐human podoplanin antibody NZ‐12 in vitro and in vivo. (a) Antibody‐dependent cellular cytotoxicity induced by human peripheral blood mononuclear cells (MNC) against malignant pleural mesothelioma cell lines ACC‐MESO‐1, ACC‐MESO‐4, NCI‐H226, and NCI‐H290/PDPN, was evaluated by 6‐h 51Cr release assay (effector/target ratio 100) in the presence of human IgG (hIgG; 1 μg/mL), NZ‐1 (1 μg/mL), NZ‐8 (1 μg/mL), and NZ‐12 (1 μg/mL), with human MNC. (b) Complement‐dependent cytotoxic activity was indicated by 6‐h 51Cr release assay in the presence of NZ‐12 (1 μg/mL) or hIgG with baby rabbit complement (1:4 dilution). (c) Human natural killer (NK) (CD56+) cells were isolated from human MNC. Antibody‐dependent cellular cytotoxic activity of NZ‐12 (1 μg/mL) mediated by human NK (CD56+) cells was evaluated by 6‐h 51Cr release assay at an effector/target ratio of 5, 10, and 25. (d, e) SCID mice (n = 5) were injected with NCI‐H290/PDPN (1.0 × 106 cells) into the thoracic cavity. NZ‐12 (100 μg) or hIgG (100 μg) injection (i.p.) was continued twice a week for 2 weeks. Human NK (CD56+) cells (1.0 × 105 cells) were injected into the thoracic cavity weekly for 2 weeks. Three weeks after tumor cell inoculation, the mice were killed and the weight of thoracic tumors (white arrows) and volume of pleural effusion were measured. *P < 0.05, **P < 0.01 (values represent mean ± SE).
