Figure 6.
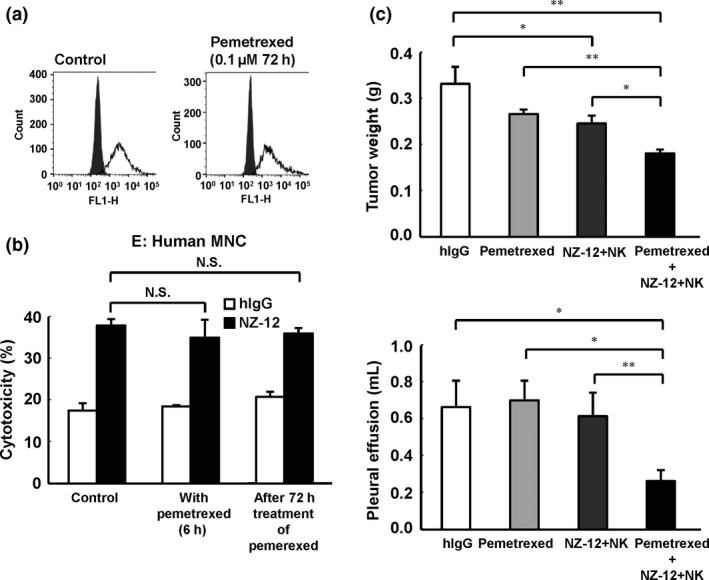
Combinatory effects of treatment for malignant pleural mesothelioma with NZ‐12‐based immunotherapy and pemetrexed in vivo. (a) NCI‐H290/PDPN was incubated with pemetrexed (0.1 μM). After 72 h of incubation, expression of podoplanin was evaluated by FACS analysis. (b) Antibody‐dependent cellular cytotoxic activity of NZ‐12 (1 μg/mL) against NCI‐H290/PDPN mediated by human peripheral blood mononuclear cells (MNC) was evaluated by 6‐h 51Cr release assay (effector/target ratio 100) in the presence or absence of pemetrexed (0.1 μM). NCI‐H290/PDPN treated with pemetrexed (0.1 μM) for 72 h was also used in target cells. (c) SCID mice (n = 5) were injected with NCI‐H290/PDPN (1.0 × 106 cells) into the thoracic cavity. NZ‐12 (100 μg) or human IgG (hIgG; 100 μg) was injected i.p. twice a week for 2 weeks. Human natural killer (NK) (CD56+) cells (1.0 × 105 cells) or normal saline was injected into the thoracic cavity weekly for 2 weeks. Pemetrexed (100 mg/kg, i.p.) was given on days 4, 5, 6, 11, 12, and 13. *P < 0.05, **P < 0.01 (values represent mean ± SE). N.S., not significant.
