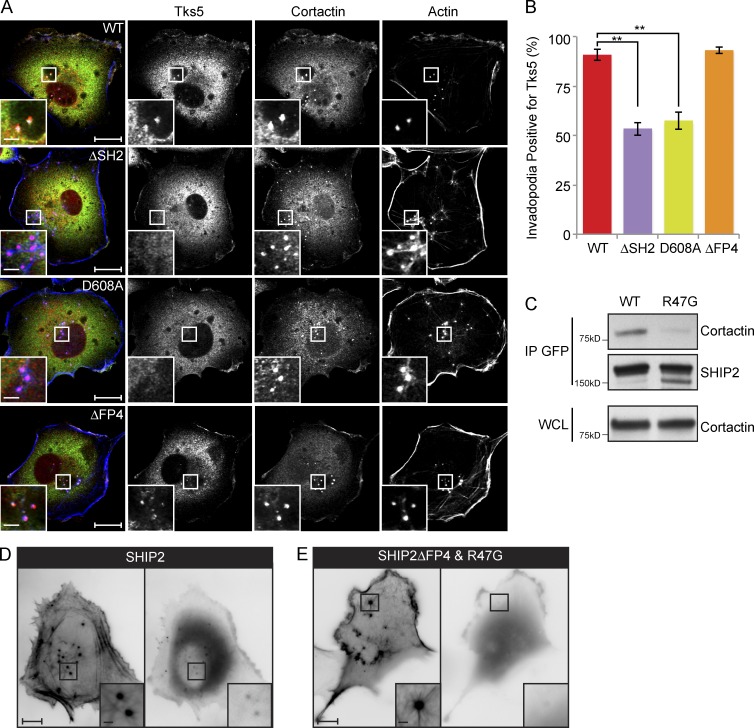
Figure 2.
SHIP2 is recruited to invadopodia via its SH2 domain, possibly through an interaction with cortactin. (A) SHIP2-depleted MDA-MB-231 cells rescued with shRNA-resistant wild-type SHIP2 or SHIP2 mutants (ΔSH2, D608A, and ΔFP4) were immunostained for invadopodia markers cortactin and Tks5, and confocal sections were acquired at the ventral surface. (B) As in A, MDA-MB-231-shSHIP2 cells rescued with WT-SHIP2, SHIP2 ΔSH2, SHIP2-D608A, or SHIP2ΔFP4 were immunostained for F-actin (phalloidin), cortactin, and Tks5. Percentage of F-actin– (phalloidin) and cortactin-positive punctae at the ventral surface of cells that were also positive for Tks5 was quantified based on three independent experiments. (C) Cell lysates were isolated from MDA-MB-231 cells transiently transfected with GFP-SHIP2 or GFP-SHIP2ΔFP4-R47G. WT-SHIP2 or SHIP2ΔFP4-R47G was immunoprecipitated using GFP antibody, and immunoprecipitates were separated by SDS-PAGE and probed as indicated. (D) MDA-MB-231 cells were transiently transfected with tagRFP-LifeAct and GFP-SHIP2 and subjected to time-lapse video microscopy analysis. Single frame depicts SHIP2 localization with respect to actin punctae. (E) MDA-MB-231 cells were transiently transfected with tagRFP-LifeAct and GFP-SHIP2ΔFP4-R47G and subjected to time-lapse video microscopy analysis. Single frame illustrates that GFP-SHIP2ΔFP4-R47G failed to localize to actin punctae. All quantified data indicate the mean values ± SE from at least three independent experiments. **, P < 0.01. Bars: 10 µm; (inset) 2 µm.