Figure 4.
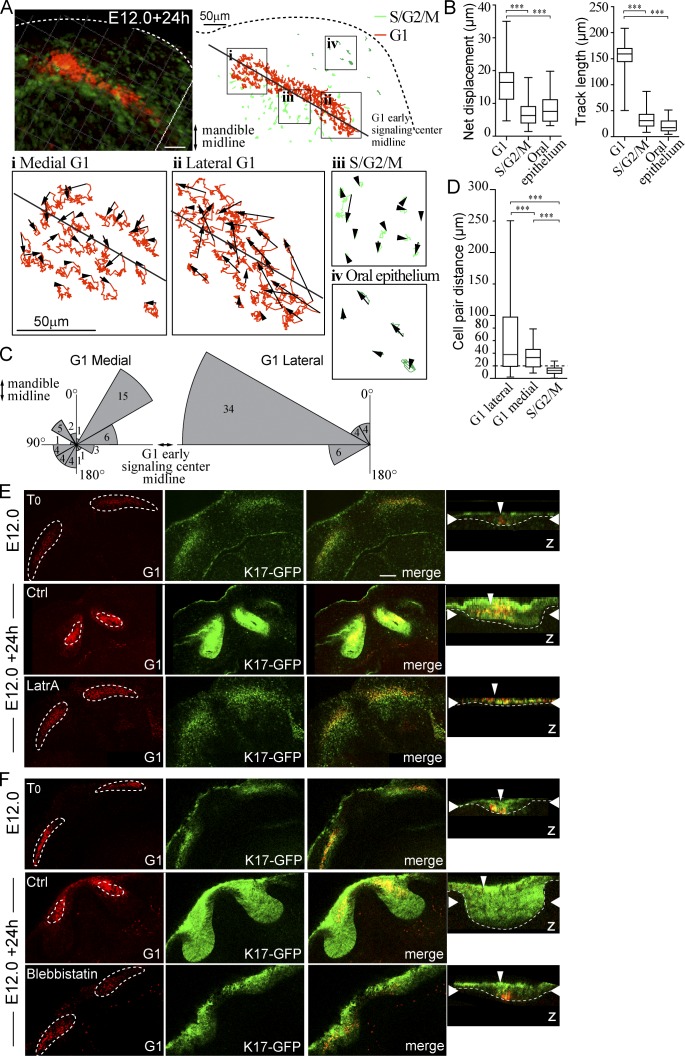
Differential cell migration contributes to early signaling center condensation. Live confocal fluorescence microscopy of Fucci transgenes in mandibular whole-mount explant was used to characterize cell movement during placode formation. Explants were imaged at E12.0 + 24 h, and movement of placode cells, adjacent S/G2/M population, and cells of the oral epithelium was tracked. (A) Cell tracks in a representative incisor in four locations: (i) medial G1, (ii) lateral G1, (iii) S/G2/M in close proximity to the early signaling center, and (iv) S/G2/M in the oral epithelium. (B) Quantification of net displacement and track length showed significantly higher values in G1 early signaling center cells (medial and lateral populations together) compared with the adjacent S/G2/M and oral epithelial cells (Student’s t test, ***, P < 0.001; nareas = 5 and ncells = 30; data shown are means ± SD). The early signaling center G1 cells exhibited higher motility with respect to other epithelial cell populations. (C) G1 cell movement angles showed persistent directional movement by the lateral cells toward the mandible midline. Medial G1 cells, in contrast, moved toward the center of the early signaling center. (D) Movement of G1 cell pairs within the forming signaling center and S/G2/M cells in the placode were studied. Cells were followed by live imaging for 24 h (E12.0 onward), and cell pairs, initially ≤15 µm distance from each other, were traced (ncell pairs = 32 in nplacodes = 3). The pairwise comparison revealed that placode cells switch their neighbors frequently in both medial and lateral populations (Student’s t test, ***, P < 0.001; mean cell pair distances = 37.7, 25.2, and 12.6, respectively). The lateral G1 pairs end at longer distances apart from each other. In contrast, S/G2/M cells retained their original neighbors. Box and whisker plot represented as minimum, 25th percentile, median, 75th percentile, and maximum values for each dataset. (E) Latrunculin A (8 µM) was applied to K17-GFP;Fucci G1 explants at E12.0 followed by 24-h culture. Initially G1 cells were distributed along the dental lamina. After 24-h control treatment, G1 cells were observed in a condensed area focus in the anterior part of the K17-GFP positive bud, similar to E13.0 incisors (compare to Fig. S1). In latrunculin A-treated explants, morphogenesis was arrested at placode stage: the signaling center did not change shape, and no invagination of the epithelium was seen. (F) As with LatrA treatment, early signaling center G1 cells stayed dispersed in samples treated with blebbistatin (100 µM), and budding morphogenesis was abrogated. Narrow vertical arrowheads indicate the position of the early signaling center and wide horizontal arrowheads show the xy optical section position in the orthogonal section. Bars, 50 µm.