Figure 2.
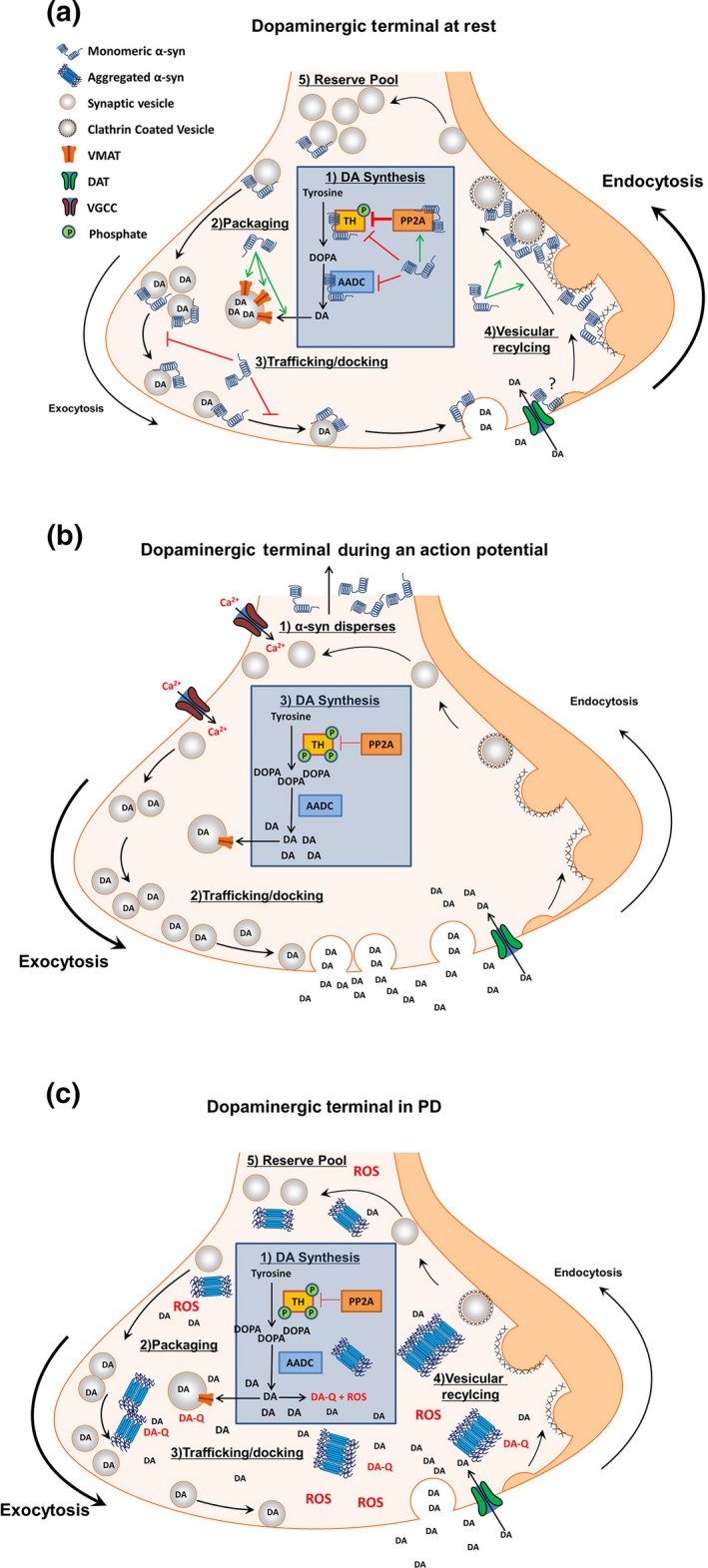
Proposed model of α‐syn function in the dopaminergic terminal. (a) In the absence of an action potential, α‐syn concentrations are high in the presynaptic terminal where it acts as a brake on chemical neurotransmission. A1) α‐syn inhibits DA synthesis by decreasing TH phosphorylation directly or by increasing PP2A activity. α‐syn also interacts with, and inhibits, AADC. A2) α‐syn aids in the sequestration of cytosolic DA by increasing the amount of VMAT on vesicles. A3) α‐syn prevents neurotransmitter release through interactions with synaptic vesicles and SNARE complex proteins to prevent trafficking and docking of vesicles with the presynaptic membrane. (A4) α‐syn facilitates the recycling of synaptic vesicles by mediating membrane bending during endocytosis, in order to (A5) maintain numbers of vesicles in the reserve (and possibly the readily releasable) vesicle pool. B1) Following neuronal stimulation and calcium influx, α‐syn rapidly disperses from the presynaptic terminal (B2) providing unimpeded vesicular trafficking and exocytosis for efficient neurotransmitter release. (B3) The absence of α‐syn disinhibits TH and AADC, allowing DA synthesis to replenish DA released during synaptic transmission. Upon repolarization, α‐syn repopulates the terminal to perform the actions listed in panel A to terminate chemical neurotransmission. (c) The aggregation of α‐syn in PD results in a loss of α‐syn function and subsequent increased cytosolic DA. (C1) Loss of α‐syn disinhibits TH and AADC, resulting in increased DA synthesis with a corresponding (C2) decrease in VMAT levels, and (C3) unregulated trafficking of synaptic vesicles. (C4) Loss of α‐syn function impairs endocytic vesicular recycling, (C5) decreasing the size of the vesicular pool. The net result is increased cytosolic DA, with a concomitant inability to efficiently sequester DA into synaptic vesicles. Increased cytosolic DA auto‐oxidizes to produce ROS and DA quinones. DA quinones react with sulfhydryl groups in proteins, forming DA‐cysteinyl adducts that covalently modify proteins, impairing enzymatic function. ROS oxidize proteins and lipids. DA‐cysteinyl adducts and ROS inhibit the electron transport chain, resulting in increased oxidative stress and opening of the mitochondrial permeability pore, as well as decreasing enzymatic break down of DA to DOPAC, further increasing cytosolic DA. Increased ROS and the formation of DA‐α‐syn adducts promote α‐syn aggregation. Together, an initial loss of α‐syn function can initiate a vicious cycle of toxicity ultimately resulting in cell death. Abbreviations: tyrosine hydroxylase (TH), aromatic amino acid decarboxylase (AADC), dopamine (DA), dihydroxyphenylalanine (DOPA), protein phosphatase 2A (PP2A), vesicular monoamine transporter (VMAT), dopamine transporter (DAT), voltage gated calcium channel (VGCC), reactive oxygen species (ROS), dopamine quinone (DA‐Q).
