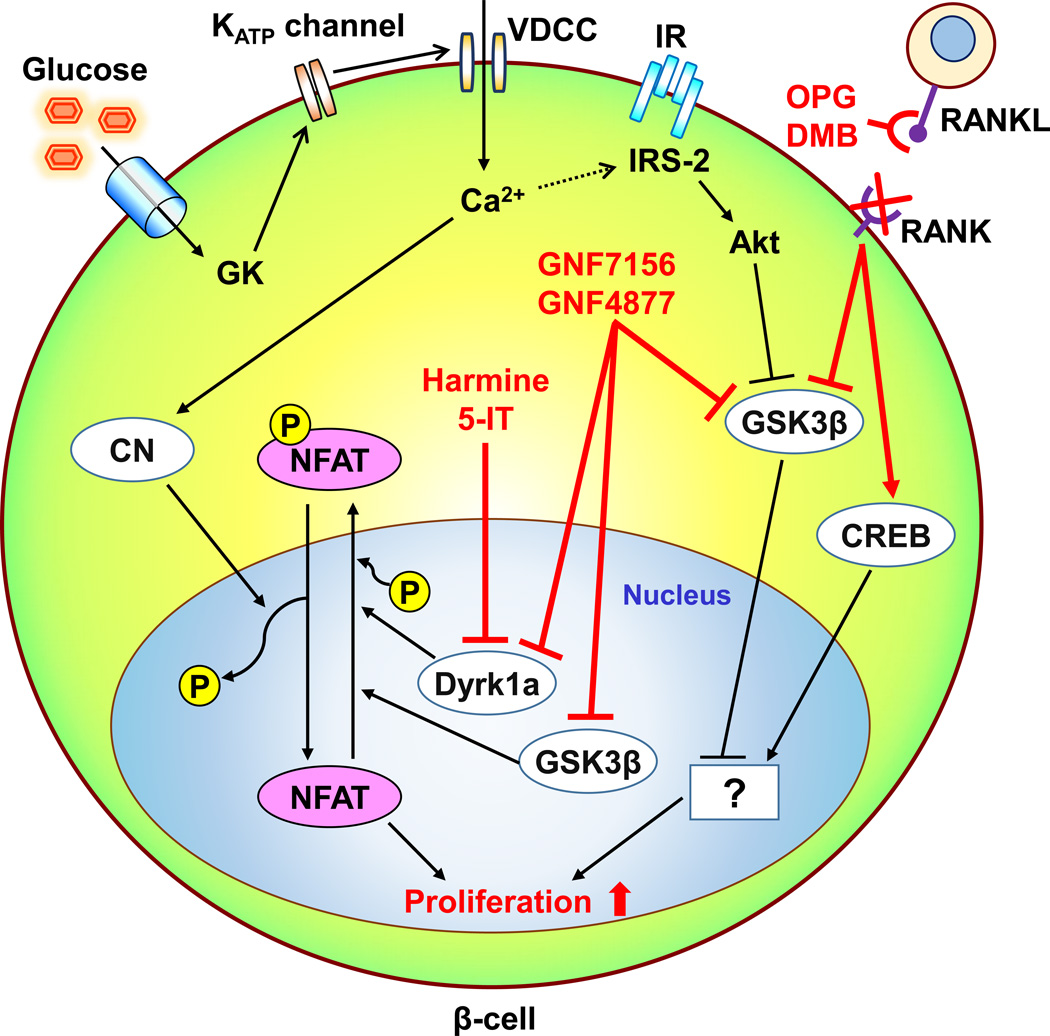
Fig. 1.
Schematic of the mechanism(s) by which recently identified factors modulate human β-cell proliferation. Glucokinase (GK)-mediated glucose signals activate human β-cell proliferation by the upregulation of intracellular calcium levels and IRS-2 mediated insulin signals. The calcium influx also activates a protein phosphatase calcineurin (CN). NFAT is translocated to the nucleus and activated by dephosphorylation with CN, while being inactivated by Dyrk1 and possibly GSK3β via its phosphorylation which results in nuclear export of NFAT. Harmine and 5-HT inhibit Dyrk1a, and GNF7156 and GNF4877 inhibit both Dyrk1 and GSK3β. These actions potentiate nuclear NFAT activation and β-cell proliferation. The phosphorylation-dependent GSK3β inactivation is also observed in insulin signal-induced Akt activation in β-cells, but the precise role of GSK3β in β-cell proliferation is still unclear. Osteoprotegrin (OPG) and Denosmab (DMG) activate CREB and inactivate GSK3β through the inhibition of an interaction between RANKL and RANK and stimulate human β-cell proliferation. However, the downstream signals of those proteins in human β-cells are unknown. CREB, cAMP response element-binding protein; DMG, Denosmab; Dyrk1A, dual-specificity tyrosine-regulated kinase-1a, GSK3β, glycogen synthase kinase-3β, IR, insulin receptor; KATP channel, ATP-sensitive potassium channel; NFAT, nuclear factor of activated T-cells, OPG, osteoprotegrin; VDCC, voltage-dependent calcium channel.