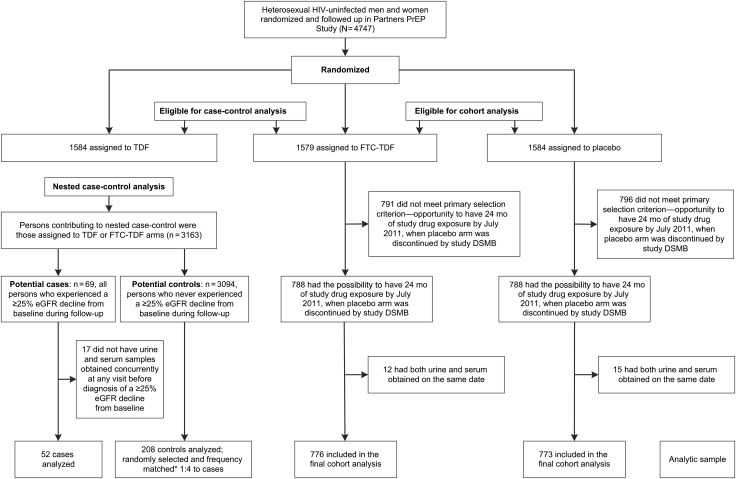
Figure 1.
Study flow diagram. We used 2 complementary designs to answer 2 related questions; (1) a cohort analysis to determine whether emtricitabine (FTC)–tenofovir disoproxil fumarate (TDF) preexposure prophylaxis (PrEP) causes proximal tubulopathy, and (2) a nested case-control analysis of participants receiving either FTC-TDF or TDF PrEP to investigate whether proximal tubulopathy predicts a subsequent ≥25% estimated glomerular filtration rate (eGFR) decline from baseline. The cohort analysis considered persons randomized to FTC-TDF and placebo in the Partners PrEP Study. The primary selection criterion for inclusion in the cohort analysis was the possibility to have the 24-month visit by 10 July 2011, when the study placebo arm was suspended by the data and safety monitoring board (DSMB); this criterion is based on a baseline variable “date of enrollment into the study” and thus preserves the randomized group assignment. Persons selected into the cohort approach (n = 1549) tended to be female and older, with lower creatinine clearance and elevated blood pressure at baseline—characteristics indicating a high propensity for kidney toxicity—compared with those not selected (n = 1614). The nested case-control analysis considered persons assigned to active PrEP arms (FTC-TDF or TDF) who experienced a ≥25% eGFR decline (case patients). Controls were persons receiving active PrEP who never experienced a ≥25% decline in eGFR and who were frequency matched to case patients by study arm (either TDF or FTC-TDF) and duration of drug exposure. Abbreviation: HIV, human immunodeficiency virus.