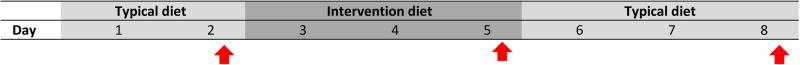Abstract
Objectives
Diet is a major source of exposure to certain phthalates, a class of environmental chemicals associated with endocrine disruption in animal models and humans. Several studies have attempted to lower phthalate exposure through carefully designed dietary interventions, with inconsistent results. We conducted a dietary intervention pilot study with the objective to lower phthalate exposure in low-income pregnant women, a particularly vulnerable population.
Methods
Ten pregnant women consumed a provided diet consisting of mostly fresh, organic foods for three days. We collected urine samples before, during, and after the intervention and conducted semi-structured interviews to assess the feasibility and acceptability of the intervention. We used repeated measures ANOVA and paired t-tests to assess differences in urinary phthalate metabolite concentrations across the study, focusing on the metabolites of di-2-ethylhexyl phthalate (DEHP), a phthalate of particular interest, and their molar sum (∑DEHP).
Results
Phthalate metabolite concentrations did not change appreciably during the intervention period. We observed no significant difference in ∑DEHP metabolite concentrations across the three time periods (F=0.21; adjusted p-value=0.65), and no reduction during the intervention as compared to baseline (t=−1.07, adjusted p-value=0.51). Results of interviews indicated that participants were not motivated to make dietary changes to potentially reduce chemical exposures outside of the study.
Conclusions
Despite the small sample size, our results suggest that promoting dietary changes to lower phthalate exposure may not be an effective public health measure. Reducing the use of phthalates in food processing and packaging may be a better solution to lowering exposure on a population level.
Keywords: phthalates, diet, prenatal exposures, endocrine disrupting chemicals, environmental chemicals
Introduction
In 2013, the American College of Obstetricians and Gynecologists and the American Society for Reproductive Medicine issued a joint statement on toxic environmental chemicals and their threat to maternal-child health (1). The statement pinpointed prenatal care as a key opportunity for providers to educate women about reducing exposure to potentially harmful chemicals during the vulnerable prenatal period. “Patient-centered actions” that may reduce chemical exposures were emphasized, including dietary changes. In some instances, dietary changes to reduce chemical exposures are empirically grounded, simple, and straight-forward to implement. For instance, limiting consumption of certain fish and switching to organic foods may effectively reduce exposure to methylmercury and pesticides, respectively (2-5). However for other chemicals, exposure reduction through diet may be considerably more complex. Phthalates are one such example. Phthalates are plasticizers found in food, as well as in consumer goods ranging from personal care products to vinyl flooring to medical devices (6). In industrialized societies, phthalate exposure is virtually universal (7-9) and in animal models and humans, prenatal exposure to some phthalates has been linked to a number of adverse health outcomes including preterm birth, altered male reproductive development, increased risk of asthma, and neurodevelopmental changes in childhood (10-20). Diet is the primary source of exposure to di-2-ethylhexyl phthalate (DEHP), a phthalate of particular interest, and fasting can dramatically reduce urinary phthalate metabolite concentrations within days (21, 22). In one study, DEHP metabolite levels fell by an order of magnitude within 24 hours of fasting (21) and in another study in which subjects were fed labelled ring-deuterated DEHP (prior to fasting), levels dropped to near or below the limit of detection with 24-hours after the initial exposure (23). Thus the terminal elimination half-life of DEHP is estimated to be approximately 4-6 hours in humans (23). Because phthalates are widely found in many types of food items (22), simple dietary changes may not be effective in lowering levels, however radical dietary transformations do result in short-term declines in phthalate metabolite concentrations (24). Phthalates are believed to enter the food supply during packaging, processing, and transport, thus choosing fresh, minimally processed foods may reduce exposure (1, 25). A small intervention study reported that consumption of a provided diet of fresh, organic foods was associated with a significant decrease in urinary DEHP metabolite concentrations (26). However a second intervention could not replicate those findings, and in fact, DEHP metabolite concentrations unexpectedly increased over 1000% from pre-intervention levels due to consumption of contaminated foodstuffs (particularly spices) (27). Also, in the second intervention study, subjects who followed a self-guided intervention (based on provided written materials, rather than provided meals) showed no drop in urinary DEHP metabolite concentrations, demonstrating the challenges of lowering dietary phthalate exposure.
We further assessed the feasibility of lowering phthalate metabolite urinary concentrations through dietary changes. Because prenatal phthalate exposure is of greatest concern, we targeted pregnant women, who are unique in their dietary requirements, restrictions, and patterns of consumption (28-30). In contrast to previous studies, which recruited participants based on self-reported consumption of canned goods and packaged foods, and took place in progressive, affluent areas (i.e., Seattle, Washington; the Bay Area, California), we examined this question in low-income, urban women, who may be least empowered to lower their chemical exposures due to lack of education and limited access to healthy, fresh foods. The objectives of this pilot study were to: (1) investigate whether phthalate metabolite concentrations decrease during a dietary intervention; and (2) explore the feasibility of women implementing similar dietary changes on their own.
Methods
Study overview
In 2013, we recruited eligible women through the University of Rochester Medical Center (URMC)'s outpatient Obstetrics and Gynecology clinic. Eligibility criteria included: seven months pregnant or less, singleton pregnancy, household income below $25,000, no major dietary limitations or allergies, and no serious threats to the pregnancy. The research subjects review board at URMC approved the study and all subjects signed informed consent before engaging in any study activities.
Following a previously used format (26, 27), subjects participated in the study for seven days (Figure 1). On day 1, subjects collected spot urine samples while following their usual diet. On days 2-4, subjects ate a provided diet, designed to be low in phthalates, and on day 4, collected a second spot urine sample, representing exposure during the intervention. On day 5, subjects returned to their usual diets and on day 7, collected a final, post-intervention spot urine sample. Throughout the study, subjects completed food logs detailing all food and beverages consumed. On days 1 and 5 (i.e. prior to and immediately after finishing the three-day intervention), subjects were interviewed.
Figure 1.
Participant schedule for dietary intervention in pregnant women. Urine samples collected on days marked by red arrows were analyzed for phthalate metabolite concentrations.
Dietary intervention
Based on the current literature on dietary sources of phthalates and menus used in similar work (26), we developed, in collaboration with URMC's Clinical and Translational Sciences Institute Bionutrition core, a menu intended to minimize dietary phthalate exposure and balanced to meet the nutritional and caloric needs of pregnant women (Appendix 1). Menus consisted of fresh and organic foods whenever possible, the latter because phthalates are sometimes included as inert ingredients in pesticides (31). The Bionutrition core purchased all foods, preparing them with stainless steel cookware and utensils, and storing them in phthalate-free plastic containers. On days 1, 2, and 3 (Figure 1), food for the following day was delivered to the subject's home. Subjects were instructed to reheat foods when necessary using a microwave and ceramic dishes, in the oven, or on a stove top using a pan (no non-stick coatings). Non-plastic plates, utensils, and cookware were provided as needed. Subjects were instructed not to use plastic water bottles during the intervention.
Aside from water, subjects were asked to eat and drink only the food and beverages provided during the three day intervention. If they needed to deviate from provided meals, they were asked to choose fresh foods or items packaged in glass, rather than plastics, and to note deviations in their daily food logs, which were used to assess compliance. This was intended solely as a dietary intervention and subjects were not asked to make any additional lifestyle modifications.
Urine sample collection and phthalate analysis
On days 1, 4, and 7, subjects were asked to collect their spot urine samples after dinner in provided phthalate-free, polypropylene urine collection cups, and to record the time of sample collection. Samples were stored in their refrigerators until the study team collected them the next day and brought them back to URMC to measure specific gravity (SG) using a refractometer (National Instrument Company, Inc., USA) . Samples were frozen at −80 °C and then sent on dry ice to the Division of Laboratory Sciences, National Center for Environmental Health, CDC for the measurement of eleven phthalate metabolites (Table 1). As described elsewhere, the analytic approach entails enzymatic deconjugation of the metabolites from their glucuronidated form, followed by automated on-line solid phase extraction, high performance liquid chromatography separation, and detection by isotope-dilution tandem mass spectrometry (32). The precision and accuracy were improved through the use of isotopically labeled internal standards.
Table 1.
Specific-gravity corrected phthalate metabolite concentrations (in μg/l) before, during, and after a 3-day dietary intervention. (n=10)
| Phthalate ester (abbreviation) | Metabolite (abbreviation) | Pre-intervention mean (±SD)a | Mid-intervention mean (±SD) a | Post-intervention mean (±SD) a | Repeated measures ANOVA F-statistic (p-value)b | LOD (% detected)c |
|---|---|---|---|---|---|---|
| Di-2-ethylhexyl phthalate (DEHP) | Mono-2-ethylhexyl phthalate (MEHP) | 2.8 (3.2) | 3.2 (2.0) | 3.6 (2.1) | 0.03 (0.88) | 0.5 (90) |
| Mono-2-ethyl-5-oxohexyl phthalate (MEOHP) | 10.0 (1.9) | 13.2 (1.8) | 12.7 (2.0) | 0.35 (0.56) | 0.2 (100) | |
| Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) | 12.2 (2.3) | 17.0 (2.1) | 18.4 (2.1) | 0.003 (0.96) | 0.2 (100) | |
| Mono-2-ethyl-5-carboxypentyl phthalate (MECCP) | 24.1 (2.0) | 30.6 (1.6) | 33.9 (2.2) | 0.39 (0.54) | 0.2 (100) | |
| ∑ DEHPd | 171.8 (19.9) | 219.6 (16.8) | 233.3 (20.6) | 0.21 (0.65) | - | |
| Di-n-butyl phthalate (DBP) | Mono-n-butyl phthalate (MBP) | 19.4 (1.6) | 24.6 (2.1) | 27.5 (1.7) | 0.32 (0.57) | 0.4 (100) |
| Diethyl phthalate (DEP) | Mono-ethyl phthalate (MEP) | 72.3 (5.2) | 125.9 (10.9) | 108.4 (4.3) | 0.70 (0.41) | 0.6 (100) |
| Di-isobutyl phthalate (DIBP) | Mono-isobutyl phthalate (MiBP) | 15.9 (1.5) | 24.0 (2.0) | 23.7 (1.5) | 0.05 (0.83) | 0.2 (100) |
| Butylbenzyl phthalate (BBzP) | Mono-benzyl phthalate (MBzP) | 10.0 (3.2) | 15.7 (2.2) | 14.2 (2.4) | 0.06 (0.80) | 0.3 (100) |
| Di-n-octyl phthalate (DnOP) | Mono-3-carboxy-propyl phthalate (MCPP)e | 4.4 (3.6) | 4.4 (2.1) | 7.0 (3.2) | 0.54 (0.47) | 0.2 (100) |
| Di-isodecyl phthalate (DIDP) | Mono-carboxy-isononyl phthalate (MCNP) | 3.7 (2.5) | 5.2 (1.5) | 5.2 (2.6) | 0.06 (0.81) | 0.2 (100) |
| Di-isononyl phthalate (DINP) | Mono-carboxy-isooctyl phthalate (MCOP) | 27.7 (3.9) | 29.4 (2.2) | 41.3 (4.5) | 0.73 (0.40) | 0.2 (100) |
Geometric mean and geometric standard deviation.
Repeated measures ANOVA examining whether there were any differences in metabolite concentrations in samples collected across the three study timepoints.
LOD= limit of detection; % detected= percentage of samples with metabolite concentrations at or above the LOD.
∑DEHP (in nmol/g) represents the sum of the DEHP metabolites (MEHP, MEOHP, MEHHP, MECCP) divided by their molecular weight.
MCPP is also a non-specific metabolite of several high molecular weight phthalates as well as a minor metabolite of DBP.
Qualitative interviews and data analysis
Two semi-structured interviews (30-60 minutes each) were conducted in the subject's home by trained investigators. In the baseline interview, women were asked about their pregnancies (e.g., attitudes, goals, concerns, health) and their typical diets. Dietary questions included items on subjects’ typical meal choices, their motivations for those choices, food sources, pregnancy-specific dietary changes, and meal preparation routines. They were also asked about what motivates their health behaviors during pregnancy (33). During the post-intervention interview, subjects were asked about the intervention including the food, comparisons to their normal eating habits, what aspects (if any) they would consider adopting in their daily lives during and after pregnancy, any obstacles or challenges, and what, if anything, would motivate them to change the way they eat during pregnancy. Interviews were audio-recorded and transcribed verbatim. Transcripts were analyzed by two independent coders using a framework analytic approach and open coding in Microsoft Word (34).
Quantitative data analysis
Phthalate metabolite concentrations below the limit of detection (LOD) were assigned as the LOD divided by the square root of two (35). Phthalate metabolite concentrations were adjusted for urine dilution using the following formula: Pc = P [(1.0225-1)/SG-1)]. In this formula, Pc is the SG-adjusted phthalate concentration (ng/ml), P is the measured phthalate concentration (ng/ml), 1.0225 is the mean SG for all study samples, and SG is the specific gravity of the individual sample (36). Four metabolites measured in our study (MEHP, MEOHP, MEHHP, MECCP) derive from the same parent compound, DEHP, thus to approximate total DEHP exposure, we calculated their molar sum (∑DEHP in nmol/ml) using the following formula (37): ΣDEHP = (MEHP*(1/278)) + (MEHHP*(1/294)) + (MEOHP*(1/292)) + (MECPP*(1/308)). Our primary outcome of interest was ∑DEHP concentrations, however secondarily, we examined concentrations of the DEHP metabolites individually, as well as concentrations of seven other phthalate metabolites.
The Shapiro-Wilk test showed that concentrations of all metabolites were non-normally distributed, and after log-transformation, all metabolites (except for MEP) followed a normal distribution. Given within-subject correlations, we performed repeated measures ANOVA F-tests (38) to assess whether there were significant differences in phthalate metabolite concentrations in the pre-, during, and post-intervention samples. We subsequently performed paired t-tests for each metabolite to test whether the mean concentration at any two of the three time points differed. Once the unadjusted p-values were computed, we applied the Benjamini-Hochberg procedure (39) to control false discovery rate. All statistical analyses were conducted using R (Version 3.1.0) (40) and a p-value of 0.05 or less was considered statistically significant.
Results
On average, the women in this pilot study were 26 years old and the group was diverse in race/ethnicity, education, marital status, and employment (Table 2). We found no significant differences in log-transformed ∑DEHP concentrations across the intervention period (F=0.21; p=0.65, adjusted p-value=0.95), nor were there pair-wise differences (pre- vs. during intervention: t=−1.07, p=0.31, adjusted p-value=0.51; during vs. post-intervention: t=−0.33; p=0.74, adjusted p-value=0.92; Table 1). We observed no significant within-subject differences in metabolite concentrations, with the exception of MiBP concentrations, which were lower pre- versus post- intervention (t=−2.74, p=0.02, adjusted p-value=0.30). Given the large number of comparisons, we cannot rule out that this significant p-value occurred by chance.
Table 2.
Characteristics of the study population (n=10)
| Mean (SD) | |
|---|---|
| Age | 26.4 (5.0) |
| Parity | 1.0 (0.8) |
| Number of months gestation | 5.5 (1.2) |
| Height (inches) | 63.1 (1.8) |
| Weight (pounds) | 173 (38.8) |
| BMI (kg/m2) | 30.6 (7.2) |
| n | |
|---|---|
| Race/ethnicity | |
| African-American (non-Hispanic) | 6 |
| African-American (Hispanic) | 1 |
| Caucasian (Hispanic) | 2 |
| Caucasian (non-Hispanic) | 1 |
| Highest level of educational attainment | |
| Less than high school | 2 |
| High school | 3 |
| Some college | 2 |
| Graduate degree | 1 |
| Marital status | |
| Married | 3 |
| Separated or divorced | 2 |
| Single | 5 |
| Employment status | |
| Employed | 7 |
| Student | 1 |
| Not employed | 2 |
| Living arrangements | |
| Home-owner | 1 |
| Rental | 7 |
| Living with family members | 2 |
In interviews, half of the women expressed dissatisfaction with the diet, and all participants indicated preferences for foods prepared with more spices and condiments. Five participants were fully compliant and the other five noted minor deviations (Appendix 2). Frequently noted barriers included cultural preferences for seasoned foods and the cost and inconvenience of fresh and organic foods (Appendix 3). Participants reported enjoying exposure to new foods, some of which they would buy for their families. Suggested strategies to overcoming barriers included: recipe books, explaining why foods are healthy, vouchers for purchasing healthy food, opportunities to try to new, unfamiliar food items, and suggestions for small changes that would improve eating habits (rather than complete dietary overhauls).
Discussion
Contra our predictions, we observed no decline in phthalate metabolite concentrations during the dietary intervention. This result contrasts with a previous study, in which DEHP metabolite concentrations were reduced by over 50% during the intervention period (26). Our results also contrast with a second study, in which DEHP metabolite concentrations spiked during the intervention period, due to use of dried herbs inadvertently “laced” with environmental chemicals (27). Given the strong evidence that diet is a major source of exposure to select phthalates including DEHP, our results suggest that our provided diet was not lower in phthalates than participants’ typical diets, despite the extensive measures taken to minimize dietary exposure during the intervention period.
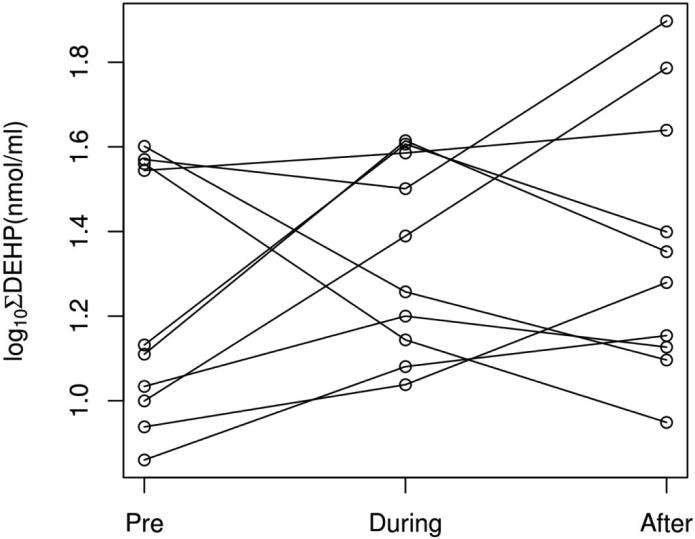
Our ability to detect changes in phthalate metabolite concentrations across the intervention period is limited by the small sample size. However the clear lack of a consistent trend towards lower concentrations during the intervention (Table 1, Figure 2) suggests that even with a larger sample size, our intervention would not have reliably lowered exposure. There are several possibilities as to why our results differed from previous work, including differences in the populations sampled (26). Whereas previous studies recruited based on consumption of canned goods and processed foods, we did not base eligibility on typical diet. To the extent that absolute phthalate metabolite concentrations are comparable across laboratories, it is worth noting that the average baseline concentrations of DEHP metabolites in the current study were at or below minimum baseline levels measured in the 10 adults in Rudel et al. (2011), while concentrations during the interventions were similar across the studies. Thus dietary interventions may be primarily effective in lowering DEHP metabolite concentrations in populations with relatively high baseline levels. Indeed, in our study, three of the four women with the highest baseline ∑DEHP concentrations experienced a decline in concentrations of these compounds during the intervention. Due to budgetary constraints, we did not measure phthalate concentrations in specific food items, therefore we cannot directly assess exposure through the provided diets, nor through other, non-dietary sources. Notably, we also did not instruct women to avoid use of plastic utensils, nor did we collect data on the types of utensils used. Therefore it is possible that there could have been contamination by use of plastic utensils, although we know of no evidence to support that as a route of exposure.
Figure 2.
Log-transformed values of the molar sum of urinary DEHP metabolites (in log[nmol/ml]) in pregnant women before, during, and after a dietary intervention. Each line represents an individual subject (n=10).
Among the six women who had the lowest ∑DEHP concentrations at baseline, concentrations were (non-significantly) higher during the intervention period. Our intervention diet was developed by a team well-versed in the exposure literature and who created menus specifically intended to reduce exposure by strictly limiting meat, dairy, spices, and processed foods. We visited multiple stores, purchased new cooking implements and servingware, and took care to minimize contamination of provided foods. Yet despite these precautions, phthalate metabolite concentrations were not lower during the intervention period. Therefore, the average woman, who may lack the time and motivation to completely change her diet, may not be able to realistically reduce her exposure to phthalates through dietary changes. This conclusion is further supported by Sathyanarayana et al.'s ineffective self-guided intervention study arm (27). Obstacles to changing women's dietary habits were further illustrated by subjects’ requests for more flavorful foods. Indeed, nearly all deviations from the provided diet were additions of salt, pepper, and seasonings (Appendix 2). There were no differences in phthalate metabolite concentrations between women who deviated from the provided diet and those who did not (not shown). Unfortunately, spices may be an important source of exposure to phthalates and little is known about brands of seasonings or condiments that may be lower in environmental chemicals (22, 27). The costs of fresh and organic foods were prohibitive for many women, most of whom relied on food stamps, and some of whom did not have regular access to a market.
Ultimately, dietary sources of phthalates may be so numerous and unpredictable that simple guidelines are currently inadequate to reduce dietary exposure during pregnancy. Testing phthalate levels in the myriad foods that women consume on a daily basis to determine which items are “safe” or “unsafe”, furthermore, is simply infeasible on a population level. Our study complicates the recent clinical messages aimed at obstetricians to advise women to limit phthalate exposure through careful food choices {ACOG, 2013 #983}. While it is important that clinicians and pregnant women recognize the potential harms that phthalates and other environmental chemicals may pose to the developing fetus, our data suggest that adopting an organic, minimally processed diet is not a simple, foolproof solution for reducing phthalate exposure. Such a diet may be beneficial for other reasons, however, phthalates are so ubiquitous that dietary changes alone appear to be insufficient for lowering exposure in most women. Given that the effects of endocrine-disrupting chemicals are “invisible” on an individual level, furthermore, women tend to express little motivation to avoid them (33). They noted that factors that might motivate them to change their diets included: (a) personal experience with health problems (e.g. childhood allergies or asthma); and (b) definitive “proof” from a reputable source (such as a government agency) that serious, adverse health effects are associated with particular exposures. The types of subtle outcomes typically linked to prenatal exposure to endocrine disruptors (e.g. decreased fecundity in adulthood) were seen as relatively unimportant and treatable. For these reasons, measures to minimize phthalate contamination in the food supply may ultimately be a more effective public health measure. Although that may be difficult given the extensive use of phthalates in food processing and packaging, there is precedent for limiting the use of phthalates in consumer products, namely toys, suggesting it can be done (41).
Supplementary Material
Acknowledgements
This study was supported by NIH grants P30 ES001247 and K12 ES019852-01. We thank the URMC CTSI Bionutrition core, particularly Pat Stewart, Nellie Wixom, and Robin Peck. We gratefully acknowledge Antonia Calafat, Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, and Tao Jia for technical assistance in measuring urinary phthalate metabolite concentrations.
Works Cited
- 1.ACOG Committee Opinion No. 575: Exposure to Toxic Environmental Agents. Obstet. Gynecol. 2013;122(4):931–5. doi: 10.1097/01.AOG.0000435416.21944.54. doi:10.1097/01.aog.0000435416.21944.54. [DOI] [PubMed] [Google Scholar]
- 2.Bjornberg KA, Vahter M, Grawe KP, et al. Methyl mercury exposure in Swedish women with high fish consumption. Sci. Total Environ. 2005;341(1-3):45–52. doi: 10.1016/j.scitotenv.2004.09.033. doi:10.1016/j.scitotenv.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Nair A, Jordan M, Watkins S, et al. Fish Consumption and Hair Mercury Levels in Women of Childbearing Age, Martin County, Florida. Maternal and child health journal. 2014 doi: 10.1007/s10995-014-1475-2. doi:10.1007/s10995-014-1475-2. [DOI] [PubMed] [Google Scholar]
- 4.Svensson BG, Schutz A, Nilsson A, et al. Fish as a source of exposure to mercury and selenium. Sci. Total Environ. 1992;126(1-2):61–74. doi: 10.1016/0048-9697(92)90484-a. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Barr DB, Pearson MA, et al. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ. Health Perspect. 2008;116(4):537–42. doi: 10.1289/ehp.10912. doi:10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC . In: Fourth Report on Human Exposure to Environmental Chemicals, 2009. Services USDoHaH, editor. Centers for Disease Control and Prevention; Atlanta, GA: 2009. [Google Scholar]
- 7.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ. Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. doi:10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112(3):331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364(1526):2063–78. doi: 10.1098/rstb.2008.0208. doi:10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005;113(8):1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Yoshinaga J, Mizumoto Y, et al. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 2012;35(3):236–44. doi: 10.1111/j.1365-2605.2011.01190.x. doi:10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 12.Gray LE, Ostby J, Furr J, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update. 2001;7(3):248–64. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Ha EH, Kim EJ, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children's Environmental Health (MOCEH) study. Environ. Health Perspect. 2011;119(10):1495–500. doi: 10.1289/ehp.1003178. doi:10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010;118(4):565–71. doi: 10.1289/ehp.0901470. doi:10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168(1):61–7. doi: 10.1001/jamapediatrics.2013.3699. doi:10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobrosly RW, Evans S, Miodovnik A, et al. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ. Health Perspect. 2014;122(5):521–8. doi: 10.1289/ehp.1307063. doi:10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornehag CG, Carlstedt F, Jonsson BA, et al. Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1408163. doi:10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson KK, McElrath TF, Ko YA, et al. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment international. 2014;70:118–24. doi: 10.1016/j.envint.2014.05.016. doi:10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien YJ, Ku HY, Su PH, et al. Prenatal Exposure to Phthalate Esters and Behavioral Syndromes in Children at Eight Years of Age: Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1307154. doi:10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whyatt RM, Perzanowski MS, Just AC, et al. Asthma in inner-city children at 5-11 years of age and prenatal exposure to phthalates: the Columbia Center for Children's Environmental Health Cohort. Environ. Health Perspect. 2014;122(10):1141–6. doi: 10.1289/ehp.1307670. doi:10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch HM, Lorber M, Christensen KL, et al. Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. International journal of hygiene and environmental health. 2013;216(6):672–81. doi: 10.1016/j.ijheh.2012.12.002. doi:10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Wormuth M, Scheringer M, Vollenweider M, et al. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803–24. doi: 10.1111/j.1539-6924.2006.00770.x. doi:10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 23.Kessler W, Numtip W, Volkel W, et al. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2- ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol. Appl. Pharmacol. 2012;264(2):284–91. doi: 10.1016/j.taap.2012.08.009. doi:10.1016/j.taap.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Ji K, Lim Kho Y, Park Y, et al. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: a pilot study with “Temple Stay” participants. Environ. Res. 2010;110(4):375–82. doi: 10.1016/j.envres.2010.02.008. doi:10.1016/j.envres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 25.RCOG Chemical exposures during pregnancy: Dealing with Potential, but Unproven, Risks to Child Health: Royal College of Obstetricians and Gynecologists. May, 2013. 2013 Contract No.: 37.
- 26.Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect. 2011;119(7):914–20. doi: 10.1289/ehp.1003170. doi:10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathyanarayana S, Alcedo G, Saelens BE, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. Journal of exposure science & environmental epidemiology. 2013 doi: 10.1038/jes.2013.9. doi:10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- 28.FDA . Food safety for pregnant women. Food and Drug Administration; 2011. Available from: http://www.fda.gov/Food/FoodborneIllnessContaminants/PeopleAtRisk/ucm312704.htm. [Google Scholar]
- 29.Kaiser L, Allen LH. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J. Am. Diet. Assoc. 2008;108(3):553–61. doi: 10.1016/j.jada.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Lowe WL, Jr., Karban J. Genetics, genomics and metabolomics: new insights into maternal metabolism during pregnancy. Diabet. Med. 2014;31(3):254–62. doi: 10.1111/dme.12352. doi:10.1111/dme.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EPA Results From Inert Ingredient Test Orders Issued Under EPA's Endocrine Disruptor Screening Program: New Data Compensation Claims; Potential Disapproval of Inert Uses Pending Public Comment. Federal Register. 2012:15101–4. [Google Scholar]
- 32.Silva MJ, Samandar E, Preau JL, Jr., et al. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2007;860(1):106–12. doi: 10.1016/j.jchromb.2007.10.023. doi:10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Barrett E, Velez M, et al. Using the Health Belief Model to illustrate factors that influence risk assessment during pregnancy and implications for prenatal education about endocrine disruptors. Policy futures in education. 2014 In press. [Google Scholar]
- 34.Gale N, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology. 2013;13:117–24. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- 36.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J. 1993;54(10):615–27. doi: 10.1080/15298669391355134. doi:10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 37.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. doi:10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis CS. Statistical methods for the analysis of repeated measurements. Springer; 2002. [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B. 1995;57:289–300. [Google Scholar]
- 40.Team RC R: A language and environment for statistical computing. 2014.
- 41.Congress HR-t, editor. GovTrack.us; 2007. Consumer Product Safety Improvement Act. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.