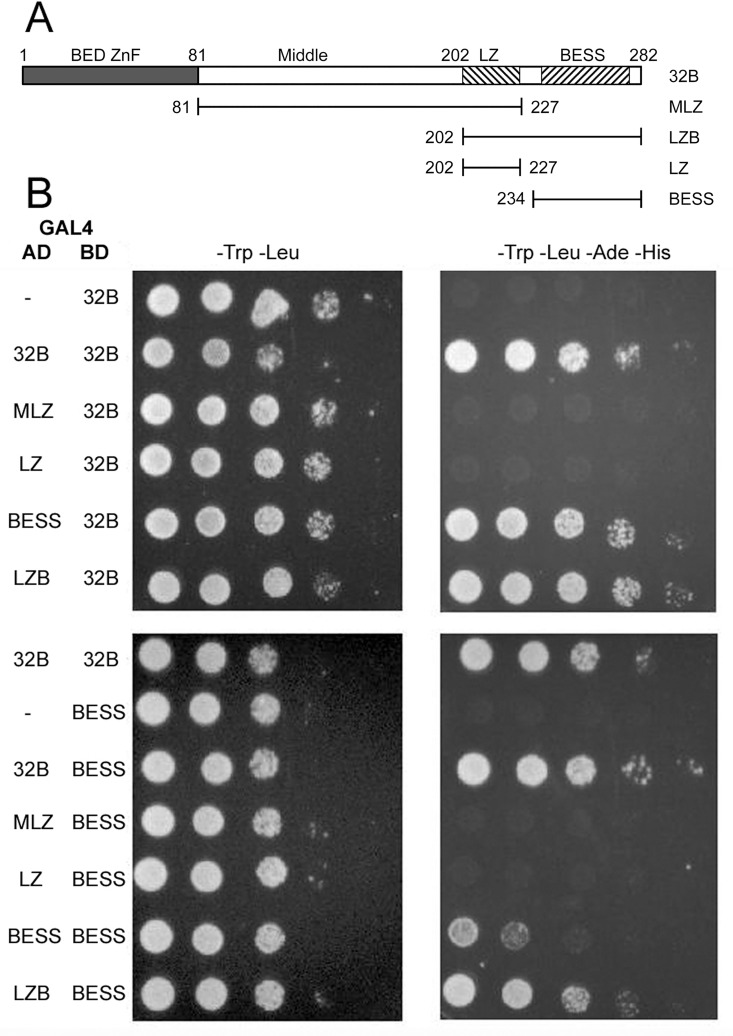
Fig 1. Yeast 2-hybrid assays demonstrate that BEAF-BEAF interactions are mediated by the BESS domain, and are strengthened if at least one protein also has the putative leucine zipper.
A. Schematic of 32B and parts derived from 32B that were fused at the carboxy ends of the GAL4 DNA-binding domain (BD) and activation domain (AD). Gray rectangle: 32B unique sequences, which encompass the DNA-binding BED finger. First hatched rectangle: putative leucine zipper. Second hatched rectangle: BESS domain. Numbers indicate the first and last amino acid present in the truncated proteins. B. Representative Y2H results, with either 32B (top panels) or the BESS domain (bottom panels) fused to the GAL4 BD. Serial 10-fold dilutions of yeast were spotted onto the plates. Left panels (-Trp -Leu) show growth on plates selecting for the presence of the BD and AD plasmids. Right panels (-Trp -Leu -Ade -His) show growth on plates selecting for the presence of the BD and AD plasmids and the expression of two reporter genes. Reporter gene expression requires that both the BD and the AD have the BESS domain (bottom right panel, BESS BESS). More vigorous growth, similar to 32B 32B and LZB 32B, was obtained if at least one protein also had the putative leucine zipper (bottom right panel, 32B BESS and LZB BESS). Similar results were obtained when the BD and AD were switched (Table 1). Results not shown here are shown in S1 Fig.