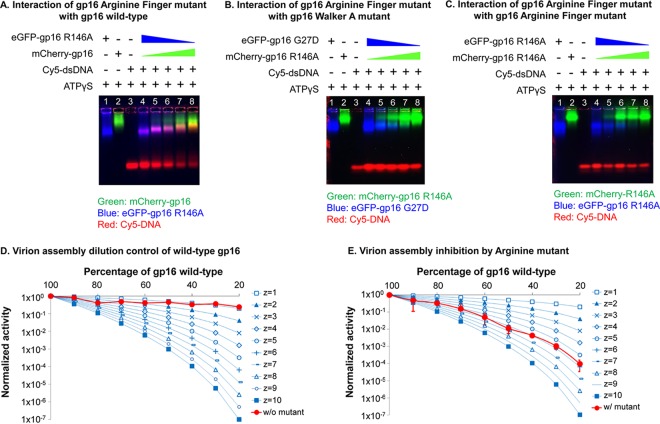
FIG 4.
Intersubunit interaction of gp16 arginine mutant with other gp16s. (A to C) EMSAs showing the interaction of the gp16 arginine finger mutant with wild-type gp16 (A), the gp16 Walker A mutant (B), and the arginine finger mutant (C). Interactions between the gp16 arginine finger mutant and wild-type gp16 or the gp16 Walker A mutant are demonstrated by the band shift of both ATPase and DNA in the gel, while no obvious band shifts were observed when the arginine finger mutant ATPases were mixed together. DNA was labeled with Cy5, and different ATPases were labeled with different fluorescent protein tags for observation in the gel. (D and E) Binomial distribution assay to show the blockage of the ATPase arginine finger mutant on motor packaging activity. Different ratios of buffer (D) or eGFP-gp16 arginine finger mutants (E) were mixed with wild-type gp16 ATPase for the in vitro virion assembly activity assay. The experimental curve is plotted with theoretical predictions made according to the equation of Fang et al. (59) The experimental curve matches with the theoretical prediction with z = 6, indicating that six subunits are present in the ATPase ring, and one arginine finger mutant is enough to block the activity of the motor (see Materials and Methods).