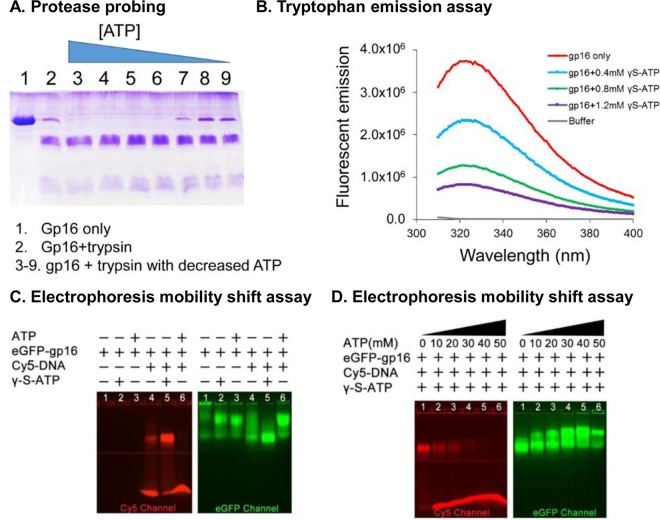
FIG 6.
Demonstration of two separate steps of gp16 conformational changes and entropic landscape alteration after ATP binding and ATP hydrolysis, respectively. (A) Trypsin probing showed that the ATPase-digested band is decreased with a reduced amount of ATP added into gp16 ATPase samples, suggesting that the gp16 ATPase is less constrained after binding to ATP. (B) Intrinsic tryptophan fluorescence assay showing the signal changes of ATPase upon the addition of different concentrations of ATP. (C) EMSA showing that gp16 ATPase bound to ATP and undergoes a conformational change that has a high affinity for DNA and that ATP hydrolysis triggers a second conformational change of gp16 ATPase with a low affinity with DNA. (D) Increasing DNA is released from gp16 ATPase/DNA/ATP complex upon the addition of increased amount of ATP that can be hydrolyzed by the gp16 ATPase.