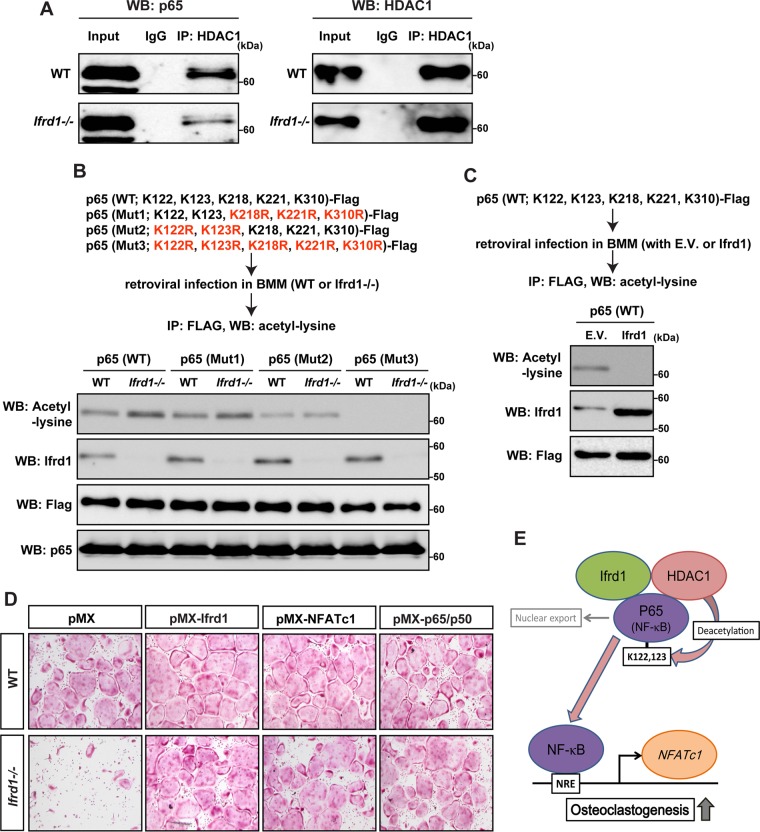
FIG 7.
Ifrd1 deficiency represses osteoclastogenesis by modulating the NF-κB/NFATc1 pathway by reducing HDAC-dependent deacetylation of p65 in osteoclasts at residues K122 and K123. Repression of interaction between HDAC1 and p65 in Ifrd1-deficient BMMs is shown. (A) BMMs from WT mice or Ifrd1−/− mice were stimulated with RANKL at 20 ng/ml and subjected to immunoprecipitation with anti-HDAC1 antibody, followed by immunoblotting with the antibodies indicated. Regulation of acetylation status of p65 at residues K122 and K123 by Ifrd1 in BMMs is shown. (B) BMMs from WT mice or Ifrd1−/− mice were infected with FLAG-tagged p65 constructs and subjected to 20 ng/ml RANKL treatment, followed by immunoprecipitation with anti-FLAG antibody and immunoblotting with the antibodies indicated. (C) WT mouse-derived BMMs were retrovirally infected with FLAG-tagged p65 (WT) vector and Ifrd1 expression vector and subjected to 20 ng/ml RANKL treatment, followed by immunoprecipitation with FLAG antibody and immunoblotting with anti-acetyl lysine antibody. Rescue of osteoclastogenesis by NFATc1 and P65/p50 in Ifrd1-deficient BMMs. (D) BMMs from WT mice and Ifrd1−/− mice were retrovirally infected with Ifrd1, NFATc1, and the p65/p50 expression vectors and subjected to stimulation with M-CSF and RANKL for 4 days, followed by TRAP staining. (E) Schematic model of this part of the study.