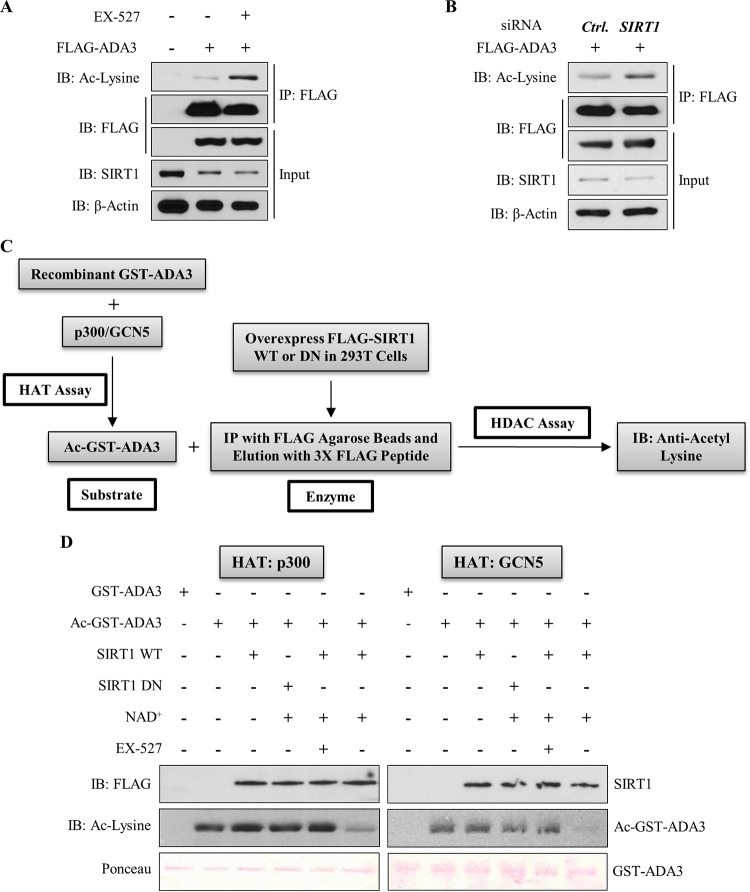
FIG 4.
SIRT1 deacetylates ADA3 in cells and in vitro. (A) HEK293T cells were transfected with FLAG-ADA3 or empty vector. Thirty hours after transfection, cells were treated either with DMSO or with 10 μM EX-527, a SIRT1-specific inhibitor, for 16 h. Cell lysates were then subjected to immunoprecipitation by M2 FLAG-agarose beads and immunoblotted with either anti-acetyl-lysine or FLAG-HRP antibody. (B) HEK293T cells were cotransfected together with FLAG-ADA3 and control (Ctrl) vector or siRNA against SIRT1. Forty-eight hours after transfection, ADA3 acetylation was analyzed by immunoprecipitation followed by immunoblotting as described for panel A. Whole-cell extracts were also immunoblotted with anti-SIRT1 and anti-β-actin antibodies to examine SIRT1 knockdown. (C) Schematic depicting the strategy used for in vitro deacetylation assay of ADA3. (D) Bacterially purified ADA3 (1 μg) was in vitro acetylated by either recombinant p300 HAT domain (25 ng) or recombinant GCN5 HAT domain (50 ng). Following this, acetylated ADA3, as a substrate, was subjected to in vitro deacetylation assay. FLAG-tagged wild-type SIRT1 or catalytic inactive mutant SIRT1 H363Y was overexpressed in HEK293T cells, immunoprecipitated by FLAG M2-agarose beads, and eluted by 3×FLAG peptide. Twenty-nanogram aliquots of these elutes were used as enzymes in the presence or absence of SIRT1 cofactor NAD+ or SIRT1 inhibitor EX-527. Following the enzymatic reaction, the reaction mixtures were subjected to SDS-PAGE and immunoblotted with anti-acetyl-lysine antibody.