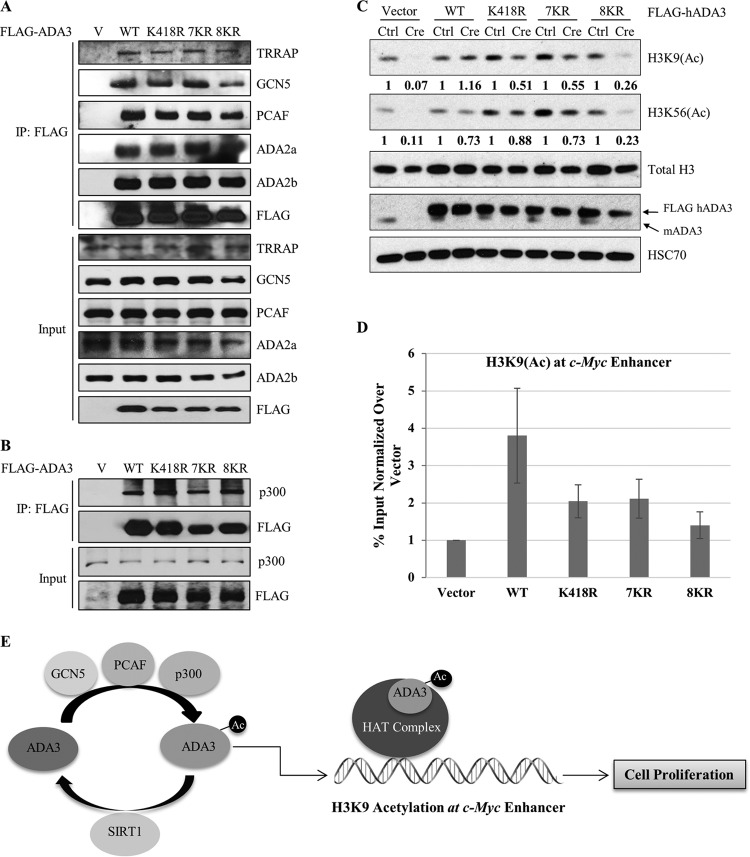
FIG 8.
Acetylation-defective mutants retain the ability to interact with various HATs and other HAT complex components yet fail to rescue the histone acetylation defects globally and at the c-Myc enhancer. (A) HEK293T cells were transfected with empty vector, ADA3 WT, or various acetylation-defective mutants. Forty-eight hours after transfection, whole-cell extracts were subjected to immunoprecipitation by M2 agarose-FLAG beads, eluted with 3×FLAG peptide, and then immunoblotted with the indicated antibodies. (B) HEK293T cells were transfected with empty vector, ADA3 WT, or various acetylation-defective mutants. Forty-eight hours after transfection, whole-cell extracts were subjected to immunoprecipitation by M2 agarose-FLAG beads and immunoblotted with the indicated antibodies. (C) Cell lysates of day 7 from cell cycle rescue experiments were immunoblotted with the indicated antibodies. The numbers underneath the blots indicate the band intensities computed from ImageJ normalized over total H3 with respect to the Ctrl. (D) A ChIP quantitative PCR of H3K9(Ac) signals at the c-Myc enhancer in Ada3-deleted MEFs overexpressing ADA3 WT or various acetylation-defective mutants. The y axis shows enrichment as a percentage of input normalized over signals in vector cells. Data represent the means ± SD from three different experiments. (E) Model showing the role of ADA3 acetylation in cell proliferation.