FIG 5.
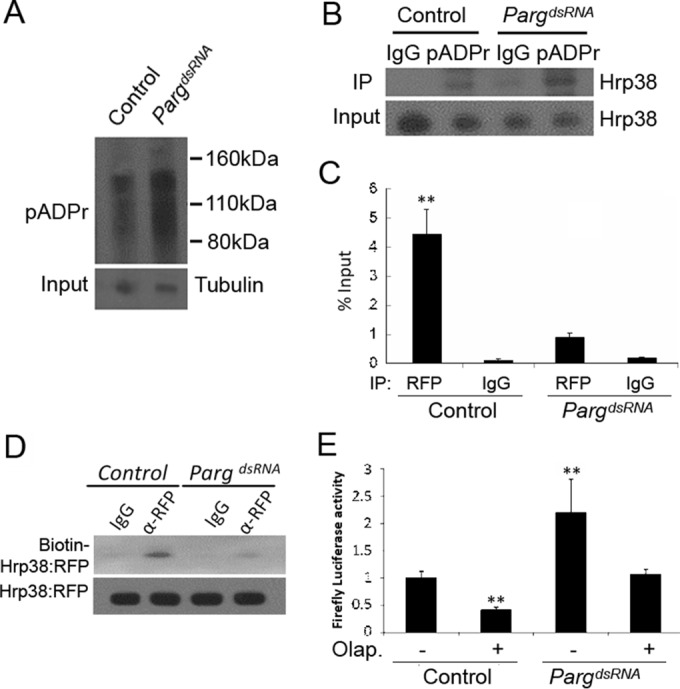
Parg RNAi enhances translation of the luciferase reporter through the inhibition of Hrp38 binding to the nos 3′ UTR. (A) Increased pADPr level in the Parg dsRNA-treated S2 cells compared to the control. Equal amounts of lysates from the control (no treatment) and Parg dsRNA-treated cells were immunoprecipitated with rabbit anti-pADPr antibody and immunoblotted with mouse anti-pADPr antibody (10H). One percent of the input was immunoblotted using antitubulin antibody for the input control. (B) Increased amounts of Hrp38 protein associated with pADPr level in the Parg dsRNA-treated S2 cells compared to the control. Equal amounts of lysates from the control and Parg dsRNA-treated S2 cells were immunoprecipitated with rabbit anti-pADPr antibody or rabbit IgG (control). The immunoprecipitates and 1% of the input were immunoblotted with rabbit anti-Hrp38 antibody. (C) Graph showing the mRNA abundance of the firefly luciferase-Nos 3′ UTR (WT) reporter associated with Hrp38:RFP after Parg dsRNA treatment. After 3 days of Parg dsRNA treatment, UAST>Hrp38:RFP, MT-Gal4, and the firefly luciferase Nos 3′ UTR reporter were transfected into S2 cells for another 3-day incubation. Quantitative RT-PCR was done to measure the mRNA level, with normalization of the input after performing RNA IP with rabbit anti-RFP antibody or rabbit IgG as a control. **, P < 0.01. (D) UV cross-linking analysis showing the decreased amounts of Hrp38:RFP protein binding to the Nos 3′ UTR in the Parg dsRNA-treated S2 cells. The protein lysate from the control or Parg dsRNA-treated cells with the expression of Hrp38:RFP was cross-linked to biotin-labeled Nos 3′ UTR (WT) RNA probe and immunoprecipitated with anti-RFP antibody. (E) Graph showing the luciferase activity of the firefly luciferase Nos 3′ UTR (WT) reporter after Parg dsRNA treatment and/or PARP1 inhibitor (olaparib [Olap.]) treatment. S2 cells were transfected with the firefly luciferase Nos 3′ UTR (WT) and the Renilla luciferase reporter after 3 days of dsRNA treatment. **, P ≤ 0.01.
