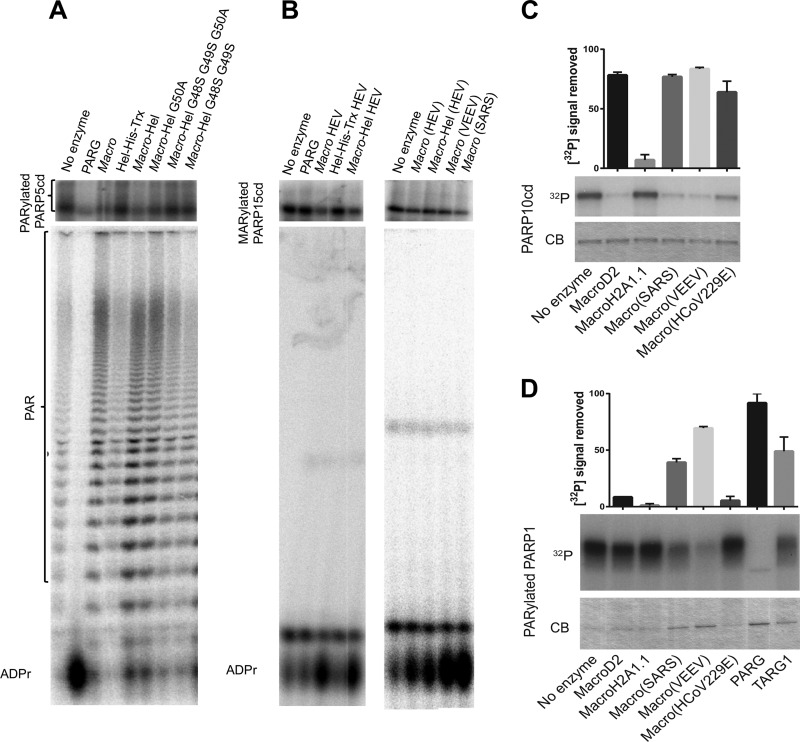
FIG 3.
Effects of HEV macro-Hel and other macro domains on PARylated PARP5 and PARP 1 and on MARylated PARP15 and PARP10. SDS–15% PAGE (upper) and 7 M urea–20% PAGE (lower) autoradiography showing the following: de-PARylation of the PARP5a catalytic domain (PARP5cd) by T. thermophila PARG, the macro domain, Hel (Hel-His-Trx), macro-Hel wild type and mutants G50A, G48S-G49S-G50A, and G48S-G49S (A); de-MARylation of the PARP15 catalytic domain by the human PARG, the macro domain, Hel, macro-Hel as well as by the VEEV and SARS macro domains on mono-ADP-ribosylated. Identification of ADP-ribose was based on comigration with cold ADP-ribose detected using UV shadowing. (C) De-MARylation of the PARP10 catalytic domain by different macro domains as observed by SDS-PAGE with Coomassie blue staining (CB) or autoradiography (32P). (D) De-PARylation of PARP1 by different macro domains as observed by SDS-PAGE with Coomassie blue staining (CB) or autoradiography (32P). Quantifications of 32P signal removed are shown above. Error bars indicate standard deviations (n = 2). ADPr, ADP-ribose.