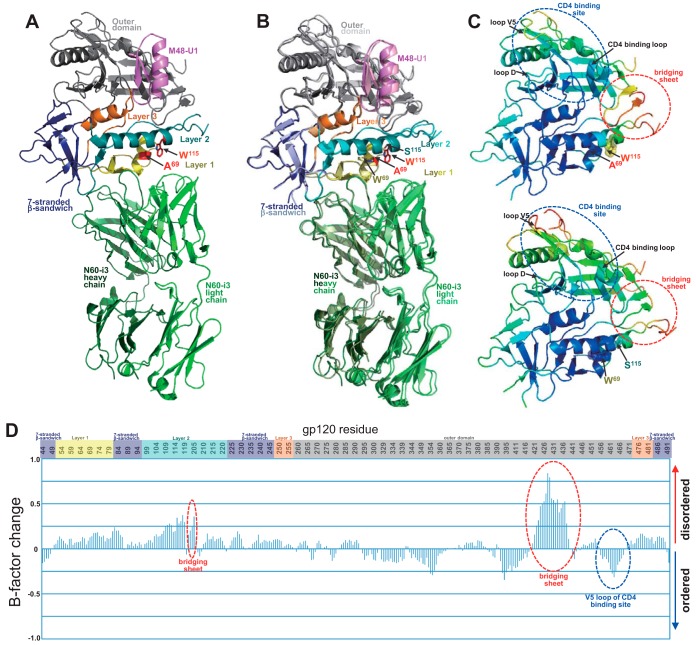
FIG 7.
Structure of monomeric gp120 (W69A/S115W) in complex with N60-i3. (A) N60-i3 Fab-g12093TH057(W69A/S115W)-M48-U1 complex ribbon diagram. N60-i3 Fab heavy and light chains are colored dark/light green, respectively. The gp120 outer domain is in gray, the 7-stranded β-sandwich in blue, mobile layer 1 in yellow, layer 2 in cyan, and layer 3 in orange. M48-U1 is in purple. Mutated residues are shown with sticks in red. (B) Superposition of the mutant N60-i3 Fab-g12093TH057(W69A/S115W)-M48-U1 complex on the wild-type N60-i3 Fab-g12093TH057-M48-U1 complex. The mutant and wild-type gp120 complexes are colored with dark/light colors, respectively, as described for panel A. Mutated residues are shown with sticks in red if mutated and with gp120 colors if wild type. (C) gp120 mutant (top) and wild-type (bottom) colors are based on the normalized B factors, with blue representing lower B factor and red higher B factor. B factors were scaled to range from 2 to 120 and the average shifted to 60 to allow comparison between the two structures. (D) Relative B factor difference for the mutant compared to wild-type gp120 scaled so that the maximum difference is 1.