Abstract
Autonomic Nervous System (ANS) balance is a key factor in homeostatic control of cardiac activity, breathing and certain reflex reactions such as coughing, sneezing and swallowing and thus plays a crucial role for survival. ANS impairment has been related to many neonatal pathologies, including Sudden Infant Death Syndrome (SIDS). Moreover, some conditions have been identified as risk factors for SIDS, such as prone sleep position. There is an urgent need for timely and non-invasive assessment of ANS function in at-risk infants. Systematic measurement of Heart Rate Variability (HRV) offers an optimal approach to access indirectly both sympathetic and parasympathetic influences on ANS functioning. In this paper, data from premature infants collected in a sleep physiology laboratory in the NICU are presented: traditional and novel approaches to HRV analyses are applied and compared in order to evaluate their relative merits in the assessment of ANS activity and the influence of sleep position. Indices from time and nonlinear approaches contributed as markers of physiological development in premature infants. Moreover, significant differences were observed as a function of sleep position.
Keywords: Autonomic Nervous System, Heart Rate Variability, Prematurity, Non-linear analysis
1. Introduction
The autonomic nervous system (ANS) is a part of the peripheral nervous system that acts to control physiology, functioning largely below the level of consciousness. It is divided in two branches, sympathetic and parasympathetic, which act synergistically. The ANS is responsible for the regulation of activity crucial for survival. It acts by maintaining homeostasis, a dynamical equilibrium that guarantees the optimal physiological function (Janig 2006). During the first months of life the development of the autonomic balance is rapidly changing: the sympathetic system develops early in pregnancy, while parasympathetic control emerges later in the perinatal period. The relative influence of parasympathetic activation increases throughout early development (Sahni et al. 2000; Longin et al. 2005) and is clearly evident when comparing neonatal and adult baseline heart rates (HR) (Gournay et al. 2002; Walker et al. 1978).
Many external conditions can affect ANS development, and also many factors related to maternal activity and metabolism can influence foetal and infant homeostatic control. For example, epidemiological studies showed that autonomic balance can be altered by prenatal factors such as alcohol and smoking exposure, and postnatal environmental influences, such as room temperature, bedding, and position during sleep (Yiallourou et al. 2011; Fifer et al. 2009).
These issues are particularly relevant for the investigation of the pathophysiology of Sudden Infant Death Syndrome (SIDS) (American Accademy of Pediatrics 1992; Guntheroth & Spiers 2002). Alterations in heart beat regulation have been identified in infants who subsequently succumbed to SIDS and these findings suggested a relationship between SIDS and autonomic control impairment (Schechtman et al. 1988; Leistner et al. 1980; Kluge et al. 1988). Many studies have explored differences in cardiovascular control in the context of risk conditions, in particular during sleep in the first months of life, in order to identify a possible fatal alteration during this vulnerable age (Sahni et al. 1999; Schechtman et al. 1988; Schechtman et al. 1990; Menihan et al. 2006; Ariagno et al. 2003; Ronald & Hannah 2010). At present however, the mechanisms leading to ANS control failure are still largely unexplained and quantification of potential risk for SIDS remains elusive.
What is clear is that the complexity underlying autonomic balance as reflected in heart rate variability (HRV), warrants further investigation and exploitation of novel analytic techniques. Indices extracted from both time and frequency domain analyses of HR have facilitated risk evaluation for pathological conditions in adult subjects (Malik et al. 1996). In this study, we employed a multi-parametric approach for the analysis of HR signals in an at-risk population, prematurely born infants. Premature birth precludes full in-utero development of the ANS and may thereby increase the probability of SIDS occurrence (Malloy 2013). As sleep position has emerged as the primary SIDS postnatal risk factor we investigated the effects of prone vs. supine position on a range of HRV parameters in this vulnerable population. Specifically, we incorporated a wide array of mathematical techniques to analyse HR signals in premature newborns collected prior to hospital discharge and then again 2 months later. This novel and comprehensive approach combined multiple indices utilizing time domain, frequency domain and nonlinear methods. Furthermore, as premature infants share physiological characteristics of the foetus and the infant, we employed a set of parameters typically employed in foetal HR analysis (Signorini et al. 2003), as well as standard adult HR studies (Malik et al. 1996).
The approach we utilized in this study also allowed investigation of several aspects of premature autonomic control that occur at different time scales, and involve different control mechanisms, known to be influenced by position and sleep state in the newborn. Moreover, the availability of a follow-up study at two months of age allowed the evaluation of the development of ANS control during the postnatal period.
2. Materials and methods
2.1. Population
The dataset was acquired in the Infant Physiology Laboratory at Columbia University Medical Center, New York. This study included data collected at two time points: the first performed on 35 healthy premature infants immediately prior to discharge from hospital and repeated in 24 of these infants at follow-up study two months after discharge. Table 1 contains subjects’ information concerning age and sex, both for the first study and the follow-up.
Table 1.
Age and sex of subject population of premature infants A) at the time of the first study right before hospital discharge and B) at the time of the follow-up study approximately 2 months after hospital discharge
| Number of subjects | Gestational Age | Post Menstrual Age |
|---|---|---|
| A) 35a | 28.7 ±2 weeks | 37.7±2.4 weeks |
| B) 24b | 27.9±1.6 weeks | 49.2±3.2 weeks |
19 males and 16 females
13 males and 11 females
Inconsistent use of the terminology employed to define newborn testing ages has made cross study comparisons problematic. We chose to define gestational age (GA) at birth as the time elapsed between the first day of the last normal menstrual period and the day of delivery and postmenstrual age (PMA) as GA plus the time elapsed since birth.
The Institutional Review Board of the Columbia University Medical Center approved the study and mothers provided informed consent at enrolment.
The duration of the first study protocol was 6 hours. During the first 3 hours infants slept in the supine position, and were then moved to prone for the rest of the study. Infants were fed right before the start of the study and again after 3 hours.
The follow up study at 2 months was designed to last one hour, due to difficulties in maintaining periods of uninterrupted sleep with older infants. Thus, infants spent only 30 minutes in each position.
Experienced clinicians coded sleep states every 30 seconds and these behavioural codes were assigned by direct observation using a scoring system previously defined and validated in the laboratory (Stefanski et al. 1984). In brief, active sleep (AS) was assigned if at least one rapid eye movement (REM) or body movement typical of AS was observed. Quiet sleep (QS) was assigned when the infant was sleeping without any observable REM activity. During QS, the infant was relaxed, with limited movements such as startles and non-nutritive sucking.
Since cardiorespiratory function is also affected by sleep state it was necessary to analyze the data within states. Nevertheless, only AS presented enough data for each baby, thus all results are summarized for AS only.
ECG was acquired at 500 Hz and the RR series in seconds were obtained using a customized peak detection algorithm, followed by visual inspection of the series to remove peaks due to arrhythmic beats or due to erroneous automated identification of R-waves.
In the first study, only patients with at least three segments of good quality RR series of 3 minutes with continuous sleep state were accepted. In the follow up, due to the reduced length of the study, patients with at least one segment of 3 minutes good quality RR series with continuous sleep state were included.
This requirement reduced the number of infants available for the analysis: for the first study 20 infants met the criteria, while for the follow up 10 infants met these criteria.
2.2. HR analysis
In this paper, techniques from time domain, frequency domain and non-linear analysis were utilized.
As there are no standardized norms developed for this particular population, we chose to adapt the guidelines developed for adults. The Task Force of 1996 suggests the evaluation of parameters on 5 minute windows for adults’ HR (Malik et al. 1996). However, since neonatal HR is higher, a similar number of beats occurs in 3 minutes time. Therefore, all parameters were evaluated on 3 minutes segments during which we allowed a maximum of 5 interruptions in the series due to noise and artefacts.
Table 2 summarizes all the parameters evaluated.
Table 2.
This table summarizes all the parameters evaluated in time domain, frequency domain and non-linear approaches, specifying the length of the sequence used to calculated them and their physiological interpretation.
| Methods and parameters for the analysis of Fetal Heart Rate | |||
|---|---|---|---|
| METHOD | PARAMETERS | SEQUENCE LENGTH | HYPOTHESIS |
| Time domain analysis: morphological HR modification and variability | SDNN (sec) | 3 min | Overall variability |
| HRVTI (sec) | Overall variability | ||
| RMSSD (sec) | Variability in the short period | ||
| LTV (bpm) | Variability in the long period | ||
| LTI (sec) | Variability in the long period | ||
| II (sec) | 1 min | Variability in the short period | |
| STV (sec) | Variability in the short period | ||
| DI (sec) | Variability in the long period | ||
| SDANN (sec) | Entire recording | Variability in the long period | |
| Frequency domain | Low frequency | 3 min | HR power for long period oscillations |
| High frequency | HR power for short period oscillations | ||
| Approximate Entropy | ApEn(m,r) m=1,2; r=0.2 | 3 min and 1500 samples | Recurrent patterns |
| Sample Entropy | SampEn(m,r) m=1,2; r=0.2 | Recurrent patterns | |
| Quadratic Sample Entropy | QSE(m,r,M) m =1,2; r=variable based on optimal count of matches (M) | Recurrent patterns | |
| PRSA | Acceleration/deceleration Phase Rectified Slope | Whole recording | Quasi-periodic oscillations |
2.2.1 Time domain analysis
For the time domain analysis it was decided to include measures adapted from adult studies’: SDNN, which is the Standard Deviation of Normal to Normal intervals, (NN), defined as the RR distances excluding anomalous, e.g. ectopic beats. In addition Standard Deviation of the Average NN interval, SDANN, the HRV triangular index, HRVTI and Root Mean of Successive Differences (RMSSD) (Malik et al. 1996) were calculated. SDNN and HRVTI estimate overall HRV, SDANN estimates long-term components of HRV and RMSSD estimates short-term components of HRV.
In addition to these traditional parameters, measures from foetal HR analyses were computed: Long Term Variability (LTV), Short Term Variability (STV), Interval Index (II), Differential Index (DI) and Long Term Irregularity (LTI) (Signorini et al. 2003; de Haan et al. 1971).
All these measures are calculated in seconds, with the exception of LTV which is defined in beats per minutes. Generally long-term variability parameters evaluate a combination of sympathetic and parasympathetic nervous systems. In contrast, measures related to the beat to beat variability are influenced largely by the parasympathetic nervous system, tied to rapid ANS reactivity.
2.2.2 Frequency domain analysis
For frequency domain analysis, each 3 minute interval of RR series was interpolated with a cubic spline and re-sampled at 10 Hz. Then Power Spectral Density was estimated with a non-parametric approach based on the Welch method, preferred for its simplicity and computational rate.
Frequency bands chosen were Low Frequency (LF), 0.05–0.2 Hz, and High Frequency (HF), 0.5–1.5 Hz (Sahni et al. 1999; Rosenstock et al. 1999). The literature does not provide precise values for these ranges, thus they were chosen based on the prior experience in our lab, on the observation of the spectra on these recordings and with consideration of the general breathing characteristics of premature infants (Sahni et al. 2000).
2.2.3 Non-linear analysis
Non-linear methods included three estimators of Entropy and the Phase-Rectified Signal Averaging (PRSA) technique.
Approximate Entropy (ApEn), as proposed by Pincus, is a the quantification of regularity, defined as the presence of repetitive patterns in a time series within a certain tolerance r and at different lags (Pincus 1991; Pincus et al. 1993). ApEn is seen as a significant analytic advancement over traditional approaches, which required very long and completely noise-free datasets to determine a precise value of entropy, neither of these demands are typically met in in vivo biological datasets. Pincus et al. advanced the field by positing that an “approximate” estimate of entropy could be used to hierarchically rank sets of time series. ApEn has been useful in separating patho-physiological conditions, but a major limitation is a strong dependence on the length of the time series analyzed and the count of the self-matches of each pattern, which is incompatible with the aim of measuring new information generated in the signal.
To address these limitations, Sample Entropy (SampEn) was developed. This new estimator introduced by Richman and Moorman (Richman & Moorman 2000), reduces the bias given by the length of the signal, does not count self-matches and enhances the estimate consistence (Lake et al. 2002).
These two methods were applied to epochs of 3 minutes duration for both the frequency domain and time domain analysis. We also analysed epochs defined by number of samples (1,500). This was decided because in epochs of 3 minutes the average number of samples is typically in the range of 400–500 and this might be too small to obtain a reliable entropy estimate (Lake 2006; Lake 2011). The tolerance r was set at 0.2 SD of the signal and the length of the pattern m varied from 1 to 3.
A challenging problem for entropy estimation is the choice of the tolerance r, to assess if two patterns could be defined similar. Lake (Lake 2006) proposed a new parameter called Quadratic Sample Entropy (QSE). It converts the measured conditional probability of SampEn to a density, simply adding the log (2r) to SampEn. The dependency on r is thus removed and r can be optimally varied for each data record, allowing direct comparison of the estimates.
Our study proposes an optimal choice of r based on the Minimum Numerator Count, which means that the r was selected if it guaranteed a minimum number of matches, in order to obtain a robust entropy estimate (Lake 2011), (Cirugeda-Roldána et al. 2014). In fact, SampEn is defined as the logarithm of the conditional probability (cp), which is the ratio of the sum of the matches found for pattern of length m +1 (A) divided by the sum of matches for pattern of length m (B), as stated in equation 1
| (1) |
In order to improve the accuracy of conditional entropy rates, the (A/B) ratio was calculated with a specified minimum number of matches for the numerator and the denominator. This algorithm guaranteed that each contribution to the total score had some minimum degree of statistical reliability.
The final step was to investigate PRSA as an alternative approach. It is a method used to synchronize the phases of quasi-periodic components of noisy and non-stationary signals, based on their temporal scale (Fanelli et al. 2013; Bauer et al. 2006). PRSA offers the ability to analyse separately HR accelerations and decelerations. This affords the opportunity to investigate rapid parasympathetic influences as well later acting sympathetic/parasympathetic ANS mechanisms. This measure requires relatively long recordings since it is based on averaging many segments to discard irrelevant data. This approach was implemented only in the initial 6 hours studies, as the duration of follow up study was deemed inadequate for this analysis.
2.3 Statistical analysis
To test for significant differences in parameters as a function of sleep position in the first study a Student’s t-test was used, because the population was normally distributed.
As the follow up population was smaller in number and not normally distributed, a Wilcoxon Signed-Rank Test was applied, a non-parametric test for populations with matched samples. This test was also applied when examining within subjects repeated measures results for the 9 subjects with data at both time points.
A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. HR as a function of PMA
Before evaluating positional differences, the behaviour of parameters as a function of PMA was verified. As a matter of fact, we already have a general knowledge of what is happening in the first months of life at the level of autonomic control, so this preliminary investigation was a way to test the performance of the proposed parameters before applying them in a less investigated topic.
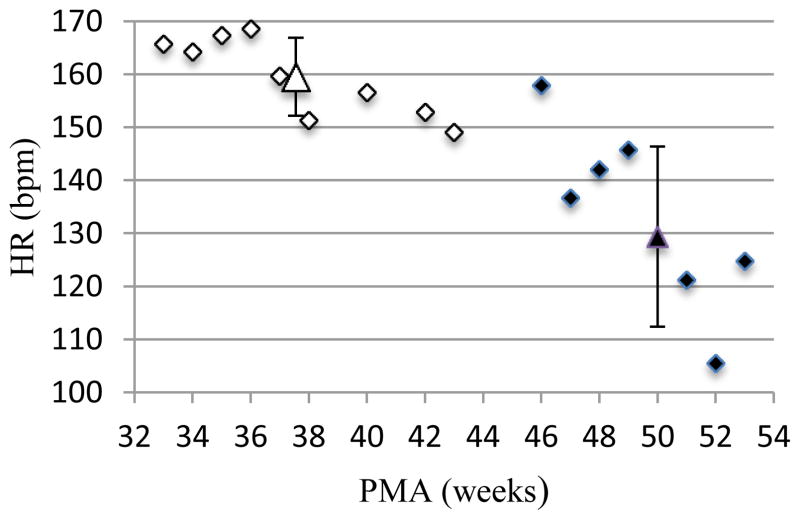
Thus, newborn infants were divided in groups according to their PMA at the time of each study. The mean HR of each group was calculated and plotted, as shown in figure 1.
Figure 1.
Infants were divided in groups by week of their post-menstrual age (PMA) at the time of the study. Then mean HR for each group was plotted against the PMA. In this figure data are plotted for infants in the first study (in white) and for those who returned for the follow up (in black). Mean and SD for each of these two groups are depicted by the large white and black triangles.
This plot shows that mean HR decrease as infants get older, as would be predicted given the growing influence of the parasympathetic system action in the first months of life. Moreover, all the variability parameters in each domain show a clear increase from the first to the follow-up study, including both short term and long-term variability measures.
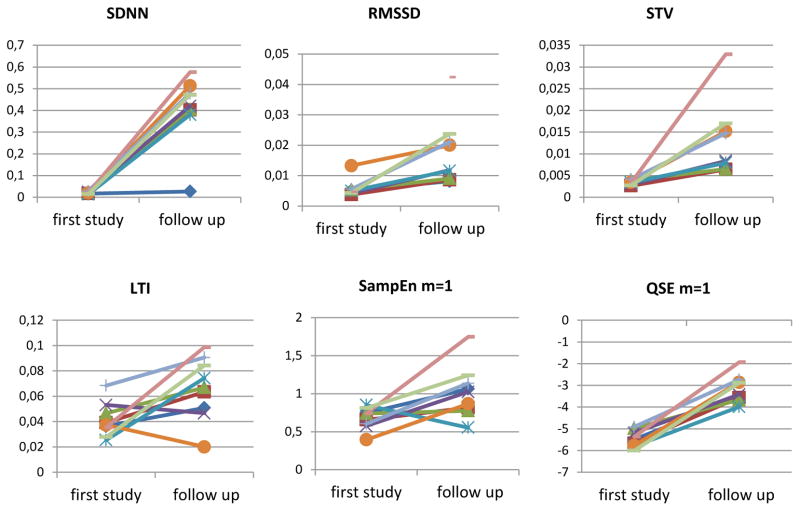
Table 3 summarizes results obtained for all parameters together with their statistical significance (p-values) and figure 2 shows the graphs of the significant parameters of the comparison.
Table 3.
Parameters’ results for the comparison of first study and follow up with patients in AS and supine position (median±IQR). P-values were obtained with Wilcoxon Signed-Rank Test on 9 subjects.
| First study | Follow up | P-val | |
|---|---|---|---|
| SDNN | 0.0182±0.006 | 0.034±0.009 | <0.01 |
| RMSSD | 0.005±0.001 | 0.012±0.01 | <0.01 |
| STV | 0.0034±0.0006 | 0.0084±0.009 | <0.01 |
| LTI | 0.0374±0.011 | 0.067±0.033 | <0.01 |
| SampEn m=1 | 0.74±0.141 | 1.023±0.321 | <0.05 |
| QSE m=1 | −5.48±0.574 | −3.42±0.71 | <0.01 |
Figure 2.
Graphs showing the comparison between the first and the follow-up study. These images illustrate augmented HRV, shown by the increase for all the parameters in the second study, as a proof of the development of the ANS during the first two months of life.
3.2. Supine vs. Prone
Analysis by sleep position in the first study showed clear differences both in the long-term variability parameters (LTI and PRSA) and in the short-term variability parameters (SampEn and QSE), with higher values in the supine position. Data for each parameter are shown in table 4.
Table 4.
Parameters’ results for first study with patients in AS (mean±SD). P-values were obtained with Student’s t-Test on 20 subjects.
| Prone | Supine | P-val | |
|---|---|---|---|
| LTI | 0.040 ± 0.018 | 0.049±023 | <0.001 |
| SDNN | 0.0212 ± 0.008 | 0.0237 ± 0.009 | <0.05 |
| PRSA ΔY | 15.66 ± 10.18 | 18.71 ± 10.68 | <0.01 |
| SampEn | 0.554 ± 0.183 | 0.443 ± 0.133 | <0.001 |
| QSE m=1 | −4.201 ± 0.415 | −4.042 ± 0.304 | <0.001 |
In the follow up study, the most robust state differences were observed only for the parameters quantifying short-term variability, specifically, RMSSD, STV and Entropies. As mentioned above PRSA could not be evaluated due to the short protocol duration at this time point. Results are shown in table 5.
Table 5.
Parameters’ results for follow up study with patients in AS (median±IQR). P-values were obtained with Wilcoxon Signed-Rank Test on 10 subjects.
| Prone | Supine | P-val | |
|---|---|---|---|
| Mean RR | 0.414± 0.048 | 0.447 ± 0.072 | <0.05 |
| RMSSD | 0.014 ± 0.009 | 0.015± 0.011 | <0.05 |
| STV | 0.01± 0.006 | 0.011 ± 0.008 | <0.05 |
| QSE m=2 | −3.27 ± 0.848 | −3.15 ± 0.783 | <0.05 |
4. Discussion
Prematurity is a brief and transitory developmental stage in which the assessment of ANS maturation may require alternative approaches and novel combinations of analytic strategies. Furthermore, parameters both from foetal and adult studies may need to be adapted as the newborn ANS is still in transition from pre- to postnatal life and thus the periodicities and the regulation of HR are operating under distinctly different physiological demands.
The goal of this study was to evaluate a wide range of HR parameters with the greatest precision possible in this vulnerable subject population, in which there is limited access and physiological control is rapidly changing.
The results from a significant subset of the parameters were in line with the current understanding of neonatal physiology. RMSSD, STV, LTI, SDNN and QSE all showed robust increases as a function of age. These measures are not simple integrated measure of variability, but they decompose variability into its components associated with relevant physiological systems. Our finding of the increased influence of the parasympathetic system during the first months of life on HR control is consistent with other developmental studies: resting state HR is reduced and the ability to respond on a beat to beat time frame is enhanced.
The second interesting finding emerged from the comparison between sleep positions, as supine position showed increased variability and also at each age a different set of parameters characterized the effect of sleep position. Specifically, prior to hospital discharge HRV differences between the two positions were found in both the long term and short-term indices of HRV, showing a reduced variability in prone position. Two months later, only short term variability indices (STV, RMSS and QSE) showed robust differences as a function of sleep position.
These results point to different autonomic regulation depending on position; this is particularly interesting in light of the fact that prone position has been identified as a major risk factor for SIDS. Furthermore the peak of SIDS events lies between the 2nd and 4th month of life, the age of the subjects in the follow up phase of our study (Yiallourou et al. 2008).
A possible explanation for the positional effects obtained in the newborn infants prior to discharge might be related to the very premature condition of the infants; their early birth is a strong stressor for their physiological development since their systems were ill prepared for the relatively early transition to extra-uterine life. Cortisol and catecholamine levels change dramatically after the 30th week of pregnancy to begin a cascade of events that would lead to labor and birth. These two mediators play a crucial role in sympathetic regulation and thus their expected maturational course may have been altered by the early delivery and physiological challenges in the NICU. In the follow up study, at two months of postnatal age, the parameters most strongly associated with the effects of sleeping position, were measures of short-term variability, generally associated with parasympathetic regulation. This might be related to the fact that at birth sympathetic activity mediated by hormonal changes is the major influence on HRV, while at two months of age parasympathetic control is more vulnerable to environmental changes.
Environmental challenges during the first months play a crucial role in the development of the parasympathetic system. Thus, the fact that in prone position the short-term variability is lower than in supine, might highlight the fact that the parasympathetic system is still not sufficiently developed to appropriately adapt to this autonomic challenge.
The number of subjects available for the study is relatively small, particularly for the maturation evaluation since only 9 babies had signals both for the first study and the follow up. Thus, our conclusions can be viewed as preliminary and an ongoing study on a bigger cohort is addressing this limitation
Spectral analysis appears to be more limited and less revealing of age and positions differences in autonomic control. One possible explanation is that such analysis is more sensitive to the neonates’ relatively high respiratory rate, which can result in cardiac aliasing. Cardiac aliasing is a phenomenon that occurs when HR is less than double the respiratory rate, and thus HR is fast enough to adjust to high respiratory movements (Harper et al. 1982; Rother et al. 1989).
5. Conclusion
This paper proposes a novel multi-parametric approach to investigate HRV to evaluate the development of ANS control in premature infants. A set of parameters both from adult and foetal HRV research contributed to our assessment of the effects of age and sleeping position.
This subset of parameters is particularly interesting because they appear to be sensitive to physiological mechanisms underlying cardiovascular control occurring under different environmental and maturational states. Since altered cardiovascular control is involved in many pathological conditions, these results seem to be promising for the development of more effective estimates of risk. Moreover, they could help in the assessment of an optimal timing for interventions aimed at optimizing the development of autonomic control during the recovery path of premature neonates.
In conclusion, a subset of parameters using a wide range of techniques, in this case LTI, RMSSD, PRSA ΔY and QSE, proved to be sensitive to the maturation of autonomic control and response to a physiological modification introduced by position and sleep state changes, offering promising tools for the evaluation of patho-physiological risk related to ANS impairment.
Acknowledgments
This work was in part supported by NIH grant R21 HD051160 to R. Sahni, R37-HD32774 to W. P. Fifer and the National Center for Advancing Translational Sciences, NIH, grant UL1 TR000040.
References
- American Accademy of Pediatrics. Positioning and SIDS. Pediatrics. 1992;89(6):1120–1126. [PubMed] [Google Scholar]
- Ariagno RL, et al. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months’ corrected age. Pediatrics. 2003;111:622–625. doi: 10.1542/peds.111.3.622. [DOI] [PubMed] [Google Scholar]
- Bauer A, et al. Phase-rectified signal averaging detects quasi-periodicities in non-stationary data. Physica A: Statistical Mechanics and its Applications. 2006;364:423–434. [Google Scholar]
- Cirugeda-Roldána EM, et al. A new algorithm for quadratic sample entropy optimization for very short biomedical signals : Application to blood pressure records. Computer Methods and Programs in Biomedicine. 2014;114(3):231–239. doi: 10.1016/j.cmpb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Fanelli A, et al. Quantitative assessment of fetal well-being through ctg recordings: A new parameter based on phase-rectified signal average. IEEE Journal of Biomedical and Health Informatics. 2013;17(5):959–966. doi: 10.1109/JBHI.2013.2268423. [DOI] [PubMed] [Google Scholar]
- Fifer WP, et al. Effects of alcohol and smoking during pregnancy on infant autonomic control. Developmental Psychobiology. 2009;51(3):234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gournay V, Drouin E, Rozé J-C. Development of baroreflex control of heart rate in preterm and full term infants. Archives of disease in childhood. Fetal and neonatal edition. 2002;86:F151–F154. doi: 10.1136/fn.86.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntheroth WG, Spiers PS. The triple risk hypotheses in sudden infant death syndrome. Pediatrics. 2002;110(5):e64. doi: 10.1542/peds.110.5.e64. [DOI] [PubMed] [Google Scholar]
- De Haan J, et al. Quantitative evaluation of fetal heart rate patterns: I. Processing methods. European Journal of Obstetrics & Gynecology. 1971;1(3):95–102. [Google Scholar]
- Harper RM, et al. Developmental patterns of heart rate and heart rate variability during sleep and waking in normal infants and infants at risk for the sudden infant death syndrome. Sleep. 1982;5(1):28–38. [PubMed] [Google Scholar]
- Janig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. New York: Cambridge United Press; 2006. [Google Scholar]
- Kluge Ka, et al. Spectral analysis assessment of respiratory sinus arrhythmia in normal infants and infants who subsequently died of sudden infant death syndrome. Pediatric research. 1988;24(6):677–682. doi: 10.1203/00006450-198812000-00005. [DOI] [PubMed] [Google Scholar]
- Lake DE. Improved entropy rate estimation in physiological data. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2011. pp. 1463–1466. [DOI] [PubMed] [Google Scholar]
- Lake DE. Renyi entropy measures of heart rate Gaussianity. IEEE Transactions on Biomedical Engineering. 2006;53(1):21–27. doi: 10.1109/TBME.2005.859782. [DOI] [PubMed] [Google Scholar]
- Lake DE, et al. Sample entropy analysis of neonatal heart rate variability. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;283:R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Leistner HL, et al. Heart rate and heart rate variability during sleep in aborted sudden infant death syndrome. The Journal of PEDIATRICS. 1980;97(1):51–55. doi: 10.1016/s0022-3476(80)80129-6. [DOI] [PubMed] [Google Scholar]
- Longin E, et al. Short term heart rate variability in healthy neonates: Normative data and physiological observations. Early Human Development. 2005;81(8):663–671. doi: 10.1016/j.earlhumdev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Malik M, Bigger J, Camm J. Heart rate variability: standards of measurement, physiological interpretations and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Malloy MH. Prematurity and sudden infant death syndrome: United States 2005–2007. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(6):470–5. doi: 10.1038/jp.2012.158. [DOI] [PubMed] [Google Scholar]
- Menihan CA, Phipps M, Weitzen S. Fetal Heart Rate Patterns and Sudden Infant Death Syndrome. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2006;35(1):116–122. doi: 10.1111/j.1552-6909.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proceedings of the National Academy of Sciences of the United States of America. 1991 Mar;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Cummins TR, Haddad GG. Heart rate control in normal and aborted-SIDS infants. The American journal of physiology. 1993;264:R638–R646. doi: 10.1152/ajpregu.1993.264.3.R638. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Ronald MH, Hannah CK. Potential Mechanisms of Failure in the Sudden Infant Death Syndrome. Curr Pediatr Rev. 2010;6(1):39–47. doi: 10.2174/157339610791317214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock EG, Cassuto Y, Zmora E. Heart rate variability in the neonate and infant: analytical methods, physiological and clinical observations. Acta paediatrica. 1999;88:477–82. doi: 10.1080/08035259950169422. [DOI] [PubMed] [Google Scholar]
- Rother M, et al. Cardiac aliasing — a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Human Development. 1989;20(1):1–12. doi: 10.1016/0378-3782(89)90068-6. [DOI] [PubMed] [Google Scholar]
- Sahni R, et al. Body position, sleep states, and cardiorespiratory activity in developing low birth weight infants. Early Human Development. 1999;54:197–206. doi: 10.1016/s0378-3782(98)00104-2. [DOI] [PubMed] [Google Scholar]
- Sahni R, et al. Maturational changes in heart rate and heart rate variability in low birth weight infants. Developmental psychobiology. 2000;37(2):73–81. doi: 10.1002/1098-2302(200009)37:2<73::aid-dev2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Schechtman V, et al. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11(5):413–424. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- Schechtman V, et al. Correlations between cardiorespiratory measures in normal infants and victims of sudden infant death syndrome. Sleep. 1990;13(4):304–317. doi: 10.1093/sleep/13.4.304. [DOI] [PubMed] [Google Scholar]
- Signorini MG, et al. Linear and nonlinear parameters for the analysis of fetal heart rate signal from cardiotocographic recordings. IEEE Transactions on Biomedical Engineering. 2003;50(3):365–374. doi: 10.1109/TBME.2003.808824. [DOI] [PubMed] [Google Scholar]
- Stefanski M, et al. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatr Res. 1984;18(1):58–62. [PubMed] [Google Scholar]
- Walker AM, et al. Sympathetic and parasympathetic control of heart rate in unanaesthetized fetal and newborn lambs. Biol Neonate. 1978;33(3–4):135–143. doi: 10.1159/000241063. [DOI] [PubMed] [Google Scholar]
- Yiallourou S, et al. The development of autonomic cardiovascular control is altered by preterm birth. Early Human Development. 2013;89(3):145–152. doi: 10.1016/j.earlhumdev.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Yiallourou SR, et al. Baroreflex sensitivity during sleep in infants: impact of sleeping position and sleep state. Sleep. 2011;34(6):725–732. doi: 10.5665/SLEEP.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiallourou SR, Walker AM, Horne RSC. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep. 2008;31(8):1139–1146. [PMC free article] [PubMed] [Google Scholar]