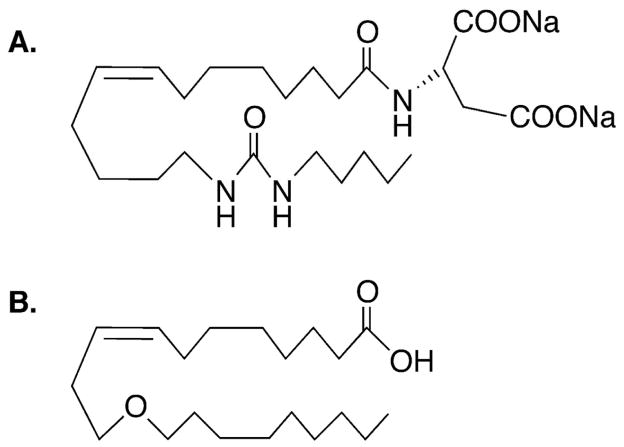
Figure 1.
(A) Chemical structure of sodium (S)-2-(Z)-(13-(3-pentyl)ureido)-tridec-8(Z)-enamido)succinate, an agonistic orally-active analog of 14,15-epoxyeicosatrienoic acid. (B) Chemical structure of 11-nonyloxy-undec-8(Z)-enoic acid (11,12-ether-EET-8ZE), an analog of 11,12-epoxyeicosatrienoic acid.