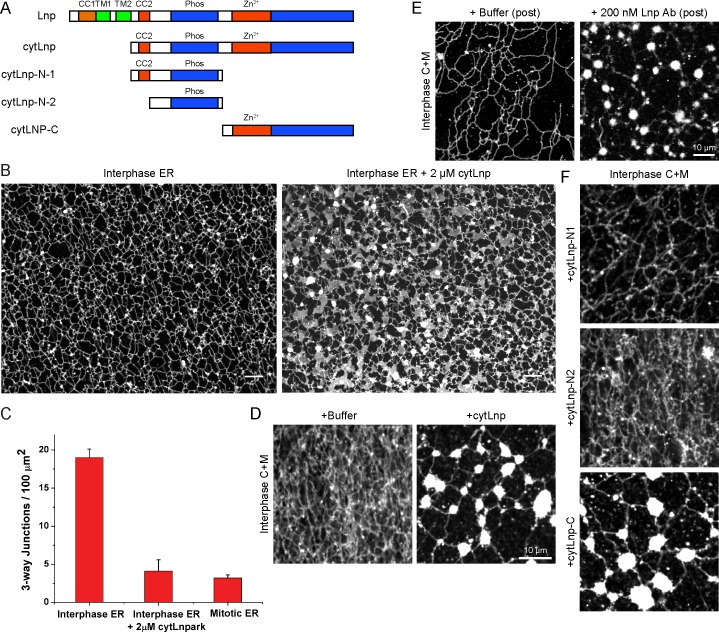
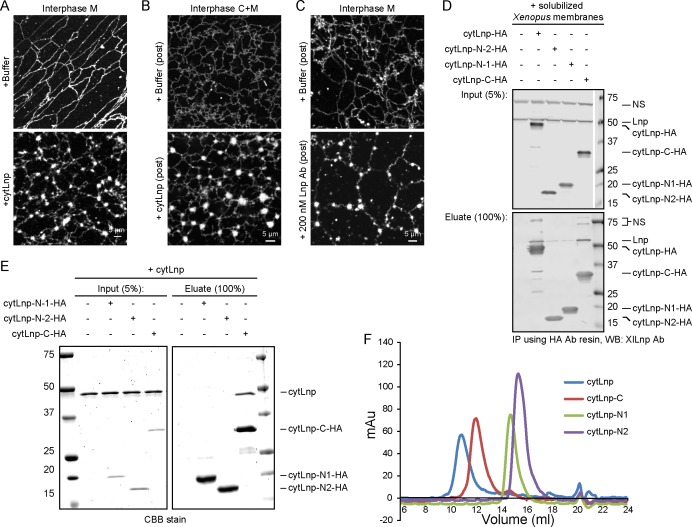
Figure 11. Effect of Lnp inactivation on an in vitro generated ER network.
(A) Schematic representation of wild type and mutant Xenopus Lnp. Phos indicates the domain phosphorylated during mitosis. (B) An ER network was generated for 20 min with crude Xenopus egg extract in the presence of the dye DiIC18 and either buffer (left panel) or 2 µM of a cytoplasmic fragment of Lnp (cytLnp). Scale bar = 10 µm. (C) The number of three-way junctions at different conditions was quantitated. Error bars indicate the mean ± SD of three independent experiments. (D) An interphase ER network was formed with Xenopus egg cytosol (C), light membranes (M), an energy regenerating system, in the presence or absence of 5 µM cytLnp. The network was stained with octadecyl rhodamine. Scale bar = 10 µm. (E) Buffer or 200 nM of affinity-purified Lnp antibodies were added to a preformed interphase network generated with cytosol, membranes, and an energy regenerating system. Scale bar = 10 µm. (F) As in (D), except that 5 µM cytLnp-N1, cytLnp-N2 or cytLnp-C were added at the beginning of the network formation reaction. Scale bars = 10 µm.