Abstract
Objective
To evaluate Plectin-1 expression as a biomarker of malignant risk for intraductal papillary mucinous neoplasms (IPMNs).
Methods
Plectin-1 immunohistochemistry (IHC) was performed retrospectively on surgical (N=71) and cytology (N=33) specimens from Mayo Clinic Jacksonville and UCLA Medical Center, including IPMNs with low grade dysplasia (LGD), high grade dysplasia (HGD), or an associated invasive adenocarcinoma.
Results
Plectin-1 expression was increased in invasive adenocarcinoma compared to adjacent in situ IPMN (p=0.005), as well as the in situ HGD component of IPMNs with invasive cancer compared to HGD of IPMNs without invasive cancer (p=0.02). Plectin IHC discriminated IPMNs with invasive adenocarcinoma from non-invasive IPMN (area under the curve [AUC] of 0.79, 75% sensitivity, 85% specificity), but was insufficient for discriminating HGD IPMN from LGD IPMNs in surgical resections (AUC 0.67, 56% sensitivity, 64% specificity) or fine needle aspiration specimens (AUC 0.45).
Conclusions
While Plectin-1 immunohistochemistry has insufficient accuracy to be used as a definitive biomarker for malignant risk in the evaluation of IPMN biopsy or cytology specimens, increased Plectin-1 expression observed in both invasive cancer and in situ HGD of malignant IPMNs suggests it might be successfully leveraged as a cyst fluid biomarker or molecular imaging target.
Keywords: Plectin-1, intraductal papillary mucinous neoplasm (IPMN), biomarker, immunohistochemistry, branch-duct IPMN, malignant progression, pancreatic adenocarcinoma
Introduction
Intraductal papillary mucinous neoplasms of the pancreas (IPMNs) are macroscopic cystic or mass-forming neoplasms arising within the pancreatic ductal system that present along a dysplastic spectrum of low grade dysplasia (LGD) to high-grade dysplasia (HGD) to associated invasive pancreatic ductal adenocarcinoma (PDA)1,2. The definitive treatment for IPMN is surgical resection. Given the potential risks associated with surgery3,4, significant efforts have been made to define robust clinical and imaging criteria that accurately differentiate malignant/high risk IPMNs requiring surgical resection from benign/low risk IPMNs that can be managed conservatively. While established guidelines are useful in stratifying risk, they still do not accurately identify all patients who do or do not require surgery 5–7, highlighting the need for additional criteria such as biomarkers to improve diagnostic accuracy8–11. One such promising biomarker is Plectin-1, previously shown to be expressed at later stages of pancreatic neoplastic progression, including high grade pancreatic intraepithelial neoplasia and most invasive PDAs 12,13, as well as in malignant IPMNs14. To further address its potential clinical utility in IPMN, Plectin-1 expression was assessed by immunohistochemistry (IHC) in a larger number of resected IPMN specimens to better define its relationship with malignant progression. We also explored the use of Plectin-1 IHC as an adjunct biomarker in endoscopic ultrasound-fine needle aspiration (EUS-FNA) samples for which definitive IPMN diagnosis and grade had been established by subsequent surgical resection.
MATERIALS AND METHODS
Samples and Data Collection
The study was performed with prior approval from the Institutional Review Boards of each participating center. Two centers were involved in the study: Mayo Clinic Jacksonville (MCJ) and University of California Los Angeles (UCLA). MCJ cases were selected from an IPMN registry prospectively maintained since 1996 and included specimens accessioned from 2006–2014. UCLA cases were identified by a pathology database search for IPMN surgical resections accessioned from 2008–2012 in the archives of the Department of Pathology and Laboratory Medicine. Demographic, clinical and radiographic information relevant to patient management including clinical symptoms, duct involvement (branch duct versus main duct), cyst size, and presence of mural nodules were collected from the clinical databases of each center.
For surgical samples, pathology reports and slides for each archival specimen were re-reviewed to confirm diagnosis and select appropriate blocks for immunohistochemical analysis. A spectrum of cases including IPMN with LGD, IPMN with HGD, and IPMN with associated invasive PDA. In instances with an associated invasive PDA, sections were chosen to simultaneously evaluate the in situ IPMN lesion and adjacent invasive PDA. For cytology samples, surgically confirmed IPMN patients from the MCJ registry who had undergone a preoperative EUS-FNA were first identified. A review of the hematoxylin and eosin cytology slides was then performed to select cases with sufficient cellularity on the cell block preparation. This prerequisite did not allow us to utilize the previously selected surgical samples due to suboptimal cellularity in most of those paired FNA samples. After appropriate FNA cases were identified, the corresponding paraffin-embedded cell blocks were obtained for slide preparation.
Immunostaining and Histologic Scoring
Plectin-1 IHC was performed using SignalStain Boost IHC Detection Reagent per manufacturer’s instructions (Cell Signaling Technology, Danvers, Mass). Detection was performed following slide deparaffinizatin and rehydration, heat-induced antigen retrieval in vegetable steamer for 20 minutes in citrate buffer (pH 6.0) and overnight incubation at 4 degrees Celsius with rabbit monoclonal anti-Plectin antibody [E398P] (ab32528, Abcam, Cambridge, Mass) at 1:600 dilution. For IHC scoring, all surgical and cytological samples were first independently reviewed by pathologists at each center (UCLA-DD; MCJ-MM, JJ) using a semi-quantitative scoring system that accounts for staining intensity (0–3, where 0 is negative, 1 is weak, 2 is moderate and 3 is strong) and percent epithelial cell staining (0 to 100%). In cases with an associated invasive PDA, the invasive PDA component and the in situ IPMN component were each scored separately. As described previously13, nerve provided an internal reference standard of moderate Plectin-1 staining intensity in surgical specimens (intensity score of 2). A final composite histoscore ranging from 0 to 12 was calculated using the intensity score (0–3) multiplied by a transformed percentage epithelial cell staining score (0–4, based on the following percentages: <5%=0, 5–25%=1, 26–50%=2, 51–75%=3, 76–100%=4). The average of scores from each observer was used for analysis. In instances of significant discrepancy in the composite histoscore (>3) between reviewers, slides were jointly reevaluated by videoconference to arrive at a final consensus histoscore.
Statistical Analysis
The Stata 13 software for Mac OS X Lion (Stata Inc, College Station, Texas) was used to manage data. The percentage, intensity and histoscore average values between centers were calculated per slide. Descriptive statistic parameters including the median and interquartile ranges were calculated for each of the three variables. The non-parametric test U Mann-Whitney was used to compare quantitative variables due to the small sample size. The receiver operating characteristic (ROC) curve was calculated to obtain the area under the curve (AUC), as well as the optimal cut-off values for the different pathological outcomes.
RESULTS
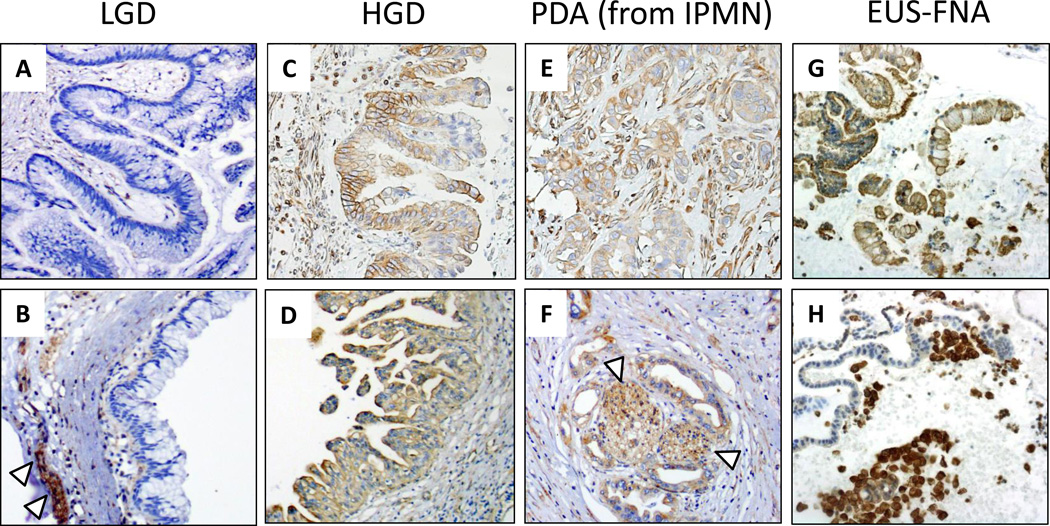
A total of 94 IPMN specimens were reviewed, including 71 surgical cases and 23 EUS-FNA cytology cases. Demographic and clinical characteristics for these cohorts are summarized in Table 1. A total of 71 surgical specimens were analyzed: 39 from MCJ and 32 from UCLA. Overall, 51 cases of non-invasive IPMNs (including 39 LGD and 12 HGD) and 20 cases with invasive PDA were evaluated, for a final tally of 91 separately generated histoscores (71 IPMN + 20 additional histoscores for the invasive PDA component of malignant IPMNs). By IHC, Plectin-1 showed heterogeneous expression in IPMNs, including both cytoplasmic and membranous staining pattern, with the presence of membranous expression typically seen in association with higher levels of cytoplasmic expression (Figure 1). IHC expression was determined semi-quantitatively using a modified histoscore accounting for staining intensity and percent tumor cell staining. Observers were in agreement (histoscore difference <3) for 65 (71%) of the scored samples. In 26 (29%) instances (6 LGD, 11 HGD and 9 invasive PDA), histoscore differences of ≥3 were resolved by group evaluation to reach a final consensus histoscore.
Table 1.
Demographic and clinical characteristics of the surgical and cytology patient cohorts
| Variable | Surgical cohort (%) | Cytology cohort (%) |
|---|---|---|
| Gender (n) | 71 | 23 |
| Male | 36 (51) | 7 (30) |
| Age (n) | 71 | 23 |
| Mean ± SD | 69 ± 10 | 69 ± 8 |
| Duct involvement (n) | 64 | 22 |
| Branch duct | 37 (58) | 13 (59) |
| Main duct | 10 (16) | 4 (18) |
| Mixed type | 17 (26) | 5 (23) |
| Cyst Size (n) | 56 | 22 |
| Mean ± SD (mm) | 31 ± 17 | 25 ± 19 |
| Mural Nodules (n) | 61 | 23 |
| Yes | 24 (39) | 8 (35) |
| Symptoms (n) | 69 | 23 |
| Yes | 38 (55) | 11 (48) |
| Type of Symptoms (n) | 38 | 11 |
| Abdominal pain | 12 (32) | 5 (46) |
| Pancreatitis | 5 (13) | 0 |
| Jaundice | 7 (18) | 1 (9) |
| Weight loss | 10 (26) | 3 (27) |
| Jaundice and weight loss | 4 (11) | 2 (18) |
Figure 1.
Representative Plectin-1 IHC is shown for (a-f) surgical resection or (g-h) EUS-FNA specimens of IPMN with LGD, IPMN with HGD, or invasive PDA arising from an IPMN. Nerve (indicated by white arrowheads) provided an internal reference standared for moderate staining intensity.
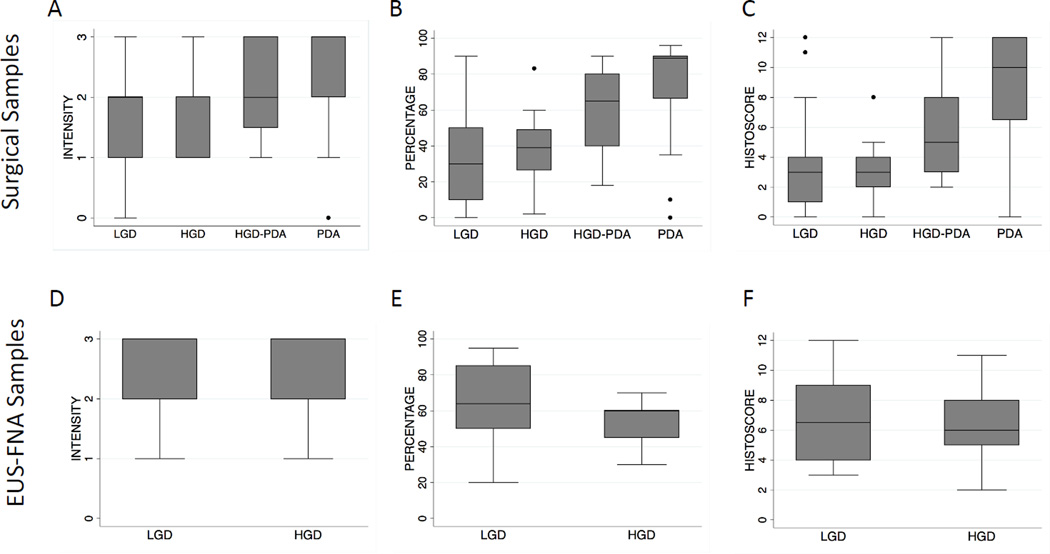
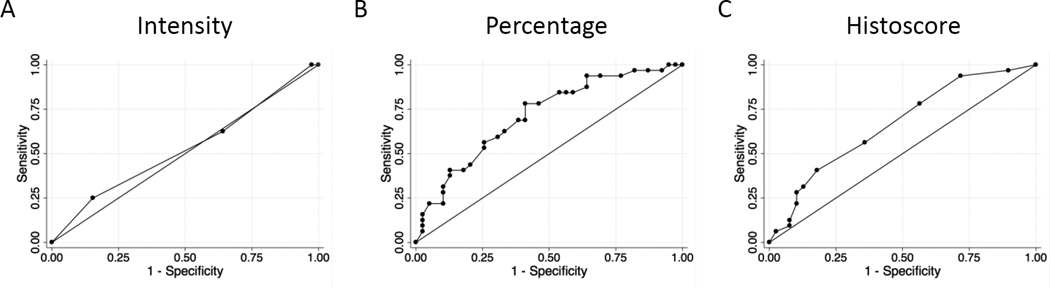
A trend of increased Plectin-1 percentage staining was observed along the histologic progression from LGD to HGD to invasive PDA (Figure 2). In contrast, staining intensity was not significantly different between LGD and HGD, but was increased in IPMNs with an associated invasive PDA (Figure 2). Although the composite histoscore did not differ between LGD versus HGD for non-invasive IPMNs, Plectin-1 was increased in the in situ HGD component of invasive IPMNs relative to LGD or HGD of non-invasive IPMNs (mean histoscore of 5.5 ± 3.3 versus 3.6 ± 2.9, p=0.02) (Figure 2). Pairwise comparison of in situ IPMN versus patient-matched adjacent invasive PDA also showed a significant trend of increased Plectin-1 staining in the invasive PDA (mean histoscore 5.5 ± 3.3 versus 8.6 ± 4.0, p=0.005, paired t-test). In receiver operating characteristic (ROC) curve analysis, Plectin-1 percentage staining and composite histoscore showed optimal diagnostic accuracy for differentiating invasive PDA associated with IPMN from those IPMNs without invasive PDA (Table 2). However, due primarily to a lack of specificity, Plectin-1 IHC showed insufficient accuracy in discriminating LGD IPMN from HGD IPMN (Figure 3 and Table 2).
Figure 2.
Semi-quantitative evaluation of Plectin-1 IHC expression for indicated grade of IPMN in (a-c) surgical resection or (d-f) EUS-FNA specimens. Box-plots show the median, interquartile range and extreme values of Plectin-1 for the individual components of (a,d) staining intensity, (b,e) percent tumor cell staining, or composite histoscore (c,f).
Table 2.
Optimal cut-off points for Plectin-1 immunohistochemistry in distinguishing different grades of IPMN in surgical specimens
| Tissue | Variable | AUC | Cutpoint | Sensitivity (%) |
Specificity (%) |
Correctly classified (%) |
|---|---|---|---|---|---|---|
| PDA vs non- PDA IPMNsa |
Intensity | 0.69 | ≥3 | 55 | 80 | 75 |
| Percentage | 0.82 | ≥58 | 80 | 72 | 74 | |
| Histoscore | 0.79 | ≥8 | 75 | 85 | 82 | |
| All HGD IPMNsb vs LGD IPMNs |
Intensity | 0.53 | ≥2 | 63 | 36 | 48 |
| Percentage | 0.71 | ≥35 | 78 | 59 | 68 | |
| Histoscore | 0.67 | ≥4 | 56 | 64 | 61 | |
| HGD onlyc vs LGD only IPMNs |
Intensity | 0.42 | ≥2 | 42 | 36 | 37 |
| Percentage | 0.60 | ≥35 | 75 | 59 | 63 | |
| Histoscore | 0.53 | ≥3 | 58 | 44 | 47 |
AUC: area under the curve;
Invasive PDA component of IPMN versus non-invasive IPMNs,
Evaluation of in situ HGD component of IPMNs with or without invasive cancer,
Evaluation of in situ HGD component with associated invasive cancer
Figure 3.
Receiver operating characteristic (ROC) curves for discriminating between HGD IPMN versus LGD IPMN using Plectin-1 IHC in surgical resection specimens based an optimal binary cut-off of (a) staining intensity, (b) percentage cell staining ≥ 35%, or composite histoscore ≥ 4.
Plectin-1 IHC was next performed on cell blocks from 23 EUS-FNA specimens, including 18 LGD and 5 HGD IPMNs. Observers were in agreement (histoscore difference < 3) for 16 (70%) of the samples. Significant differences in histoscores for 7 cases (30%, including 5 LGD and 2 HGD) were resolved by group evaluation to reach final consensus score. Plectin-1 IHC intensity, percentage and overall histoscores did not differ significantly between LGD and HGD IPMN in EUS-FNA samples (Figure 2). Likewise, Plectin-1 histoscore lacked diagnostic accuracy in ROC analysis for distinguishing LGD and HGD IPMN in EUS-FNA material (AUC=0.45, Supplemental Table 1).
We next addressed whether there was a correlation between Plectin-1 IHC expression and established factors used to clinically assess malignant risk in IPMN, including: involvement of the main duct, cyst size, presence of mural nodules, and clinical symptoms. No significant correlations were observed in either the surgical or the cytology cohorts (p>0.20, Supplemental Table 2). We next addressed the utility of Plectin-1 IHC specifically in the subset of branch-duct IPMNs in the surgical cohort (n=43) using an optimal histoscore cut-off of 4 or greater. Although Plectin-1 IHC alone had insufficient accuracy in distinguishing between malignant BD-IPMN and benign BD-IPMN in ROC analysis (AUC=0.75, 67% sensitivity, 64% specificity; Supplemental Table 3), it demonstrated sensitivity and specificity similar to established clinical criteria used to assess risk (Table 3). When considered in combination with these clinical criteria, Plectin-1 histoscore reduced overall sensitivity but improved specificity and positive predictive value for BD-IPMN with HGD or invasive PDA (Table 3).
Table 3.
Accuracy of Plectin-1 IHC and established clinical variables for detecting high risk BD-IPMN.
| Test | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Plectin | 67 | 64 | 57 | 72 |
| Size >30mm | 67 | 56 | 52 | 70 |
| Mural Nodule | 53 | 67 | 53 | 67 |
| Symptoms | 72 | 68 | 62 | 77 |
| Plectin+Size>30mm | 50 | 84 | 69 | 70 |
| Plectin+Mural Nodule | 47 | 88 | 73 | 70 |
| Plectin+Symptoms | 50 | 92 | 82 | 72 |
Plectin-1 IHC histoscore (cut point ≥4) was used to dichotomize malignant risk alone or in combination with indicated clinical variables.
DISCUSSION
Present clinical and imaging criteria for assessing malignant risk in IPMN are not wholly accurate5–7 and could be improved through the further use of robust molecular or protein-based biomarkers. Our findings here and those of prior studies indicate that increased expression of Plectin-1 is a molecular harbinger of malignant progression that might be suitably leveraged for the clinical assessment of IPMN either prior to and after surgery. Plectin-1 is a complex high-molecular weight protein and important member of the cytolinker or plakin family of proteins15,16. Expressed in a wide variety of tissues, Plectin-1 anchors intermediate filaments to membrane proteins and physically regulates various key cytoskeletal components17,18, maintaining the structural integrity and cytoskeletal architecture of cells and serving as a scaffolding platform for the positioning and regulation of signaling complexes19–21. Plectin-1 was first proposed as a PDA biomarker after its identification in a phage display screen of peptides able to bind PDA cell surface antigens12. Subsequently, Bausch, et al., demonstrated Plectin-1 IHC expression in patient tissue samples at later stages of dysplastic progression, including invasive PDA (100%), PanIN 3 (60%) and rarely PanIN 2 (4%), with no expression observed in normal pancreas, chronic pancreatitis or PanIN1. For that cohort, Plectin-1 IHC accurately discriminated PanIN 3 and PDA from normal pancreas, chronic pancreatitis or PanIN 1 with a sensitivity of 87% and specificity of 98%13.
We observed no significant difference in Plectin-1 IHC expression between LGD and HGD in non-invasive IPMNs, and Plectin-1 expression lacked sufficient sensitivity and specificity (56% and 64%, respectively) to discriminate HGD from LGD in our cohort. In contrast, a previously published study reported Plectin-1 expression accurately distinguished benign (low or moderate grade dysplasia) from malignant IPMNs (HGD and invasive PDA) with sensitivity and specificity of 83% and 84%14. This discrepancy in part reflects the way in which patient groups were segregated for analysis in each study. Indeed, consolidating high grade IPMN with or without invasive PDAC into one group versus benign IPMN improved the diagnostic accuracy of Plectin-1 (AUC 0.72), with some increase in sensitivity (65%) and no change in specificity (64%). It should also be noted that our study examined Plectin-1 expression in a far larger number of benign IPMNs (39 versus 6 in the previous study) and employed a semi-quantitative scoring system. The prior study dichotomized Plectin-1 expression as positive or negative based on the presence of dysplastic epithelial cells staining intensity equal or greater than that of nerve. Further technical issues specific to our study include possible variations in staining arising from differences in handling and processing of specimens at two institutions, although this concern is offset by the technical reproducibility of immunohistochemical staining at both institutions and use of nerve as an internal reference standard to account for variations between individual samples. We also noted some interobserver variability for the IHC scoring, highlighting that any clinically deployed IHC would require the development of reliable reference standards and reproducible scoring system to reduce such interobserver variability. Plectin-1 staining also did not differentiate between LGD and HGD IPMNs (AUC of 0.45) in EUS-FNA cytology specimens with a further concern that weak Plectin-1 staining was observed in non-pancreatic contaminants, including gastric and duodenal epithelial cells (data not shown). The paucicellular nature of most EUS-FNA specimens is a further factor limiting the use Plectin-1 as an IHC-based biomarker for diagnostic cytology.
In this study Plectin-1 expression discriminated the in situ HGD component of IPMNs with an associated invasive PDA versus HGD of IPMNs without invasive PDA, a finding of importance when considering how Plectin-1 might be used as biomarker for assessing malignant risk in IPMNs. Plectin-1 fulfills many characteristics of an ideal molecular biomarker for early PDA detection as outlined in a recent summative review of early PDA detection22, including its altered pattern of expression associated with the progression of incipient to early invasive cancer. While not observed in all high grade PanIN or IPMN lesions, Plectin-1 expression appears uniformly increased in invasive PDA13, as well as in the HGD component of cancer-associated IPMN. This suggests increased Plectin-1 expression is influenced by or directly involved at the later stages of stepwise progression from high grade PanIN or IPMN to invasive PDA. Offering one explanation, Shin, et al, recently showed PDA cells produce exosomes containing Plectin-1, leading to its cellular redistribution, extracellular trafficking and presence in the circulation and with the ability to promote tumor growth and cancer cell invasion23. Such trafficking of Plectin-1 from cancer cell-derived exosomes provides one viable explanation for increased Plectin-1 expression in the in situ HGD epithelium, as well as cyst fluid, of invasive IPMNs. It is also notable that Plectin-1 could be immunoprecipitated from cyst fluid samples of a small number of malignant, but not benign, IPMNs in a previous study13. To the extent that percentage of Plectin-1 staining in dysplastic lining epithelium provided the discriminatory power of generated histoscores in our study and the fact that core biopsy or cytology specimens are prone to sampling bias, quantitative cyst fluid measurements of Plectin-1 likely represent the most sensitive and reliable assay to leverage its use as a diagnostic marker. Our efforts are now focused on the development of a robust ELISA for Plectin-1 and the establishment of quantitative thresholds that can be applied to a robust diagnostic assay in pancreatic cyst fluid samples. Likewise, the high sensitivity and specificity of increased Plectin-1 for invasive PDA in both the setting of PanIN and IPMN warrants its continued development as a molecular imaging target or theranostic for pancreatic adenocarcinoma.
Supplementary Material
Acknowledgments
Funding: DWD was supported by grants from the American Cancer Society (RSG-12-083-01-TBG), NIH (P01 CA163200 and P01 DK098108) and the Hirshberg Foundation for Pancreatic Cancer Research. HCC was supported by NIH R01 CA159222. MM and MW were supported by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville, Florida.
Footnotes
Disclosure: K.A.K. is affiliated with iTi Health, where a plectin targeting peptide imaging agent is licensed. All other authors declare no direct conflict of interest in relation to the present study..
BIBLIOGRAPHY
- 1.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 2.Z’Graggen K, Rivera JA, Compton CC, et al. Prevalence of activating Kras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491–500. doi: 10.1097/00000658-199710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 4.Swanson RS, Pezzi CM, Mallin K, et al. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer database. Ann Surg Oncol. 2014;21:4059–4067. doi: 10.1245/s10434-014-4036-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee KH, Lee SJ, Lee JK, et al. Prediction of malignancy with endoscopic ultrasonography in patients with branch duct-type intraductal papillary mucinous neoplasm. Pancreas. 2014;43:1306–1311. doi: 10.1097/MPA.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 6.Del Chiaro M, Segersvard R, Pozzi Mucelli R, et al. Comparison of Preoperative Conference-Based Diagnosis with Histology of Cystic Tumors of the Pancreas. Ann Surg Oncol. 2014;21:1539–1544. doi: 10.1245/s10434-013-3465-9. [DOI] [PubMed] [Google Scholar]
- 7.Goh BKP, Thng C-HT, Tan DMY, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg. 2014;208:202–209. doi: 10.1016/j.amjsurg.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–227. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2015 doi: 10.1016/j.gie.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Springer S, Wang Y, Molin MD, et al. A Combination of Molecular Markers and Clinical Features Improve the Classification of Pancreatic Cysts. Gastroenterology. 2015;149:1501–1510. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Chen DT, Fulp WJ, et al. Plasma MicroRNAs as Novel Biomarkers for Patients with Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancer Prev Res (Phila) 2015;8:826–834. doi: 10.1158/1940-6207.CAPR-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KA, Bardeesy N, Anbazhagan R, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bausch D, Thomas S, Mino-Kenudson M, et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17:302–309. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bausch D, Mino-Kenudson M, Castilloc Fernández-del, et al. Plectin-1 is a Biomarker of Malignant Pancreatic Intraductal Papillary Mucinous Neoplasms. J Gastrointest Surg. 2009;13:1948–1954. doi: 10.1007/s11605-009-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- 16.Okumura M, Uematsu J, Hirako Y, et al. Identification of the hemidesmosomal 500 kDa protein (HD1) as plectin. J Biochem. 1999;126:1144–1150. doi: 10.1093/oxfordjournals.jbchem.a022560. [DOI] [PubMed] [Google Scholar]
- 17.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Andrä K, Lassmann H, Bittner R, et al. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997;11:3143–3156. doi: 10.1101/gad.11.23.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andra K, Nikolic B, Stocher M, et al. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osmanagic-Myers S, Gregor M, Walko G, et al. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174:557–568. doi: 10.1083/jcb.200605172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. doi: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih SJ, Smith JA, Rezniczek GA, et al. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:19414–19419. doi: 10.1073/pnas.1309720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.