Figure 1.
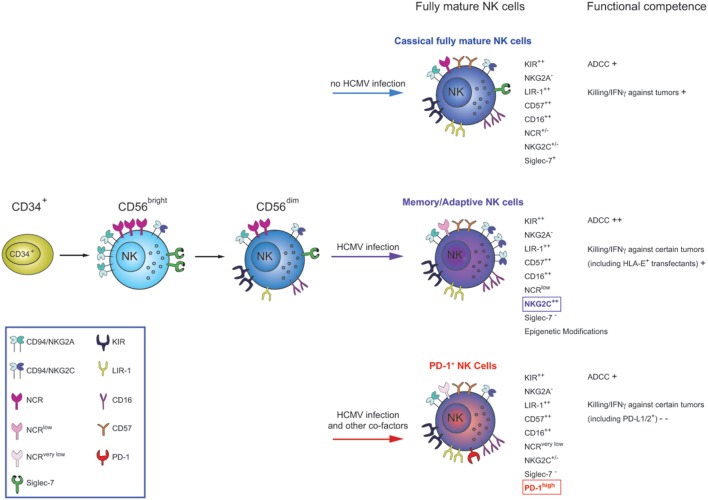
NK cells differentiate starting from CD34+ bone marrow precursors into CD56bright (CD94/NKG2A+KIR−) and then to CD56dim cells. CD56dim NK cells, in turn, continue to differentiate throughout their life-span, acquiring novel functional and phenotypic properties. During this process, they lose expression of CD94/NKG2A, sequentially acquire inhibitory KIRs and, at the final step, CD57. This molecule appear to define a subpopulation of highly differentiated NK cells (classical fully mature NK cells), characterized by the KIR+LIR-1+NKG2A−NCR+/− phenotype. Functionally, these cells display natural cytolytic activity and ADCC against tumor targets, but poor responsiveness to cytokine stimulation. After HCMV infection/reactivation, increased proportions of a subset of terminally differentiated CD57+ NK cells, characterized by high expression of NKG2C and downregulation of Siglec-7 receptors are induced (the so-called memory-like NK cells). These cells display increased functional capability in terms of ADCC and IFN-γ production/killing in response to HLA-E+ and opsonized HCMV-infected targets but decreased function after cytokine stimulation. Following HCMV infections accompanied by other cofactors (infections?), an additional type of CD57+ NK cell subset can be generated. This subset is characterized by the expression of the inhibitory PD-1 receptor (not necessary co-expressed with NKG2C) and a very low expression of the NCRs, NKp30 and NKp46. These cells, called PD-1+ NK cells, are characterized by compromised effector functions against tumor cells expressing ligands for PD-1 as well as against tumors primarily killed on NCRs/NCR-ligands interactions.