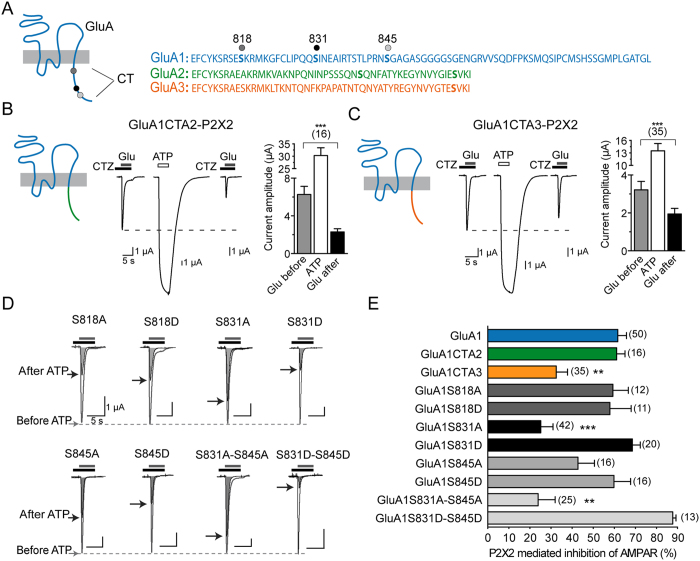
Figure 2. The carboxy tail of the GluA1 and Ser831 residue is necessary but not sufficient for P2X-mediated AMPAR depression.
(A) Schematic of AMPAR subunit topology and sequence alignment of the intracellular carboxy-terminal tails (CT) of GluA1-A3 subunits. The three main phosphorylation sites on GluA1 known to contribute to synaptic plasticity are indicated by dots. (B,C) Chimeric GluA1 receptors with the intracellular CT of either GluA2 (B) or GluA3 (C) subunits were designed to determine the region involved in the inhibitory effect of P2X2 activation. Representative currents evoked by applications of glutamate (Glu 1 mM, 5 s) in the presence of cyclothiazide (CTZ, 100 μM, 10 s of preincubation) before and 2 min after an ATP-induced current (100 μM) in oocytes co-expressing P2X2 and chimeric GluA1CTA1 or GluA1CTA3 receptors. The mean amplitudes of currents are also indicated. (D) Superimposed glutamate-evoked currents before and after ATP-induced P2X2R current recorded in the same conditions as in (B) for point or double GluA1 mutants. Ser818, Ser831 and Ser845 were mutated into alanine (A) or phosphomimetic aspartate residues (D). Maximal amplitude after ATP-induce currents are indicated by black arrows. (E) Summary bar graph representing the percentage of P2X2-mediated AMPAR current inhibition for wild-type, chimeric and mutated GluA1 receptors. Statistical differences compared to GluA1 are indicated. **p < 0.01; ***p < 0.001 number of cells is indicated between brackets.