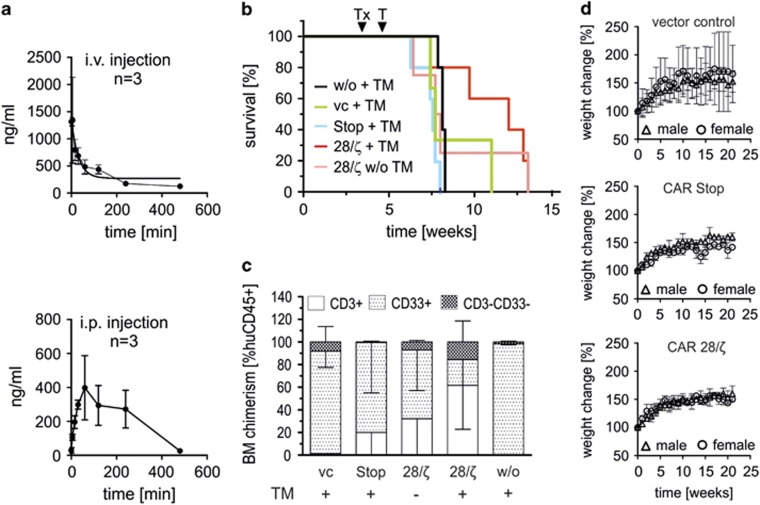
Figure 3.
UniCAR engineered T cells delay AML engraftment in a TM-dependent manner in vivo. (a) Pharmacokinetics of the dual-specific anti-CD123/CD33 TM (αCD123-CD33 TM) in peripheral blood of NSG mice upon i.v. injection via the tail vain or i.p. injection. Concentration of TM in blood samples was determined by ELISA. (b, c) Short-term treatment with anti-CD123-CD33 TM enhances the survival of NSG mice in an aggressive AML model. 1 × 106 human T cells engineered to express functional UniCARs (28/ζ+TM, red line, n=5), UniCARs lacking any signaling domain (Stop+TM, blue line, n=5) or expressing only enhanced green fluorescent protein (EGFP) marker protein (vc+TM, green line, n=3) were i.v. injected into NSG mice. After 28 days, 5 × 105 MOLM-13 were transferred into NSG mice via i.v. injection (Tx) and treatment with anti-CD123-CD33 TM (T) was started 5 days later. For this purpose, 250 ng TM per g mouse body weight was injected i.p. twice a day over 2 consecutive days. As additional controls, one group of mice was transplanted only with MOLM-13 and treated with anti-CD123-CD33 TM (w/o+TM, black line, n=4), and another group of mice was transplanted with functional UniCAR T cells and MOLM-13, but not treated with anti-CD123-CD33 TM (28/ζ w/o TM, light red line, n=4). (b) Survival curves of experimental groups and (c) percentage of CD3+ T cells, CD33+ MOLM-13 cells and human CD45+CD3-CD33- cells in the bone marrow of killed mice are shown. (d) To evaluate in vivo toxicity of UniCAR modified T cells, 1 × 106 engineered human T cells were i.v. injected into NSG mice. Body weight of mice was monitored in weekly intervals and expressed as percentage body weight change over time.