Abstract
Objectives
Evaluation for a potentially life-threatening cardiac event in the emergency department (ED) is a stressful experience that can result in symptoms of posttraumatic stress disorder, which are associated with increased risk of morbidity and mortality in patients. No study has tested whether good clinician-patient communication in the ED is associated with better psychological outcomes in these individuals and whether it can mitigate other risk factors for posttraumatic stress symptoms (PSS) such as perception of life threat and vulnerability in the ED.
Methods
Data were analyzed from 474 participants in the REactions to Acute Care and Hospitalization (REACH) study, an observational cohort study of ED predictors of medical and psychological outcomes after evaluation for suspected ACS. Participants were recruited from November 2013 to January 2015 at a single site academic medical center (New York-Presbyterian-Columbia University Medical Center). Participants reported threat perceptions in the ED and provided information on their perceptions of clinician-patient communication using the Interpersonal Process of Care Survey. PSS were assessed using the Acute Stress Disorder Scale during follow-up.
Results
474 subjects were enrolled in the study. Median length of follow-up was 3 days after ED presentation, range 0–30 days, 80% within 8 days. Perceptions of good clinician-patient communication in the ED was associated with lower PSS whereas increased threat perception was associated with higher PSS. A significant interaction between clinician-patient communication and threat perception on PSS suggested that patients with higher threat perception benefited most from good clinician-patient communication.
Conclusion
Our study found an association between good clinician-patient communication in the ED during evaluation for potentially life-threatening cardiac events and decreased subsequent posttraumatic stress reactions. This association is particularly marked for patients who perceive the greatest degree of life threat and vulnerability during evaluation.
Introduction
Patients who present to the emergency department (ED) with symptoms indicative of acute coronary syndrome (ACS) experience a great deal of stress.[1–2] Evaluation for non-ST elevation myocardial infarction (NSTEMI) or unstable angina (UA) in the ED can be accompanied by feelings of fear, vulnerability, and loss of control.[3] Indeed, a recent meta-analysis found that 12% of ACS patients subsequently screen positive for posttraumatic stress disorder because of the experience, and that elevated posttraumatic stress symptoms (PSS) after ACS are associated with increased risk for recurrent cardiac events and mortality.[4] ED variables such as crowding have been associated with the development of PSS,[5] but no study has tested whether good clinician-patient communication can offset risk for PSS.
Clinician-patient communication has been found to have a significant impact on multiple patient health outcomes, including anxiety, depression, treatment adherence, physical functioning, and overall patient satisfaction.[6–7] [8–10] [11] In the ED, good clinician-patient communication may be protective against the development of PSS, particularly for patients with the highest levels of perceived threat. Emergency care providers may be uniquely capable of reducing the uncertainty and fear that accompany evaluation for ACS through clear and compassionate communication with the patient in the acute context of the disease.
In this study, we examined the association of patients’ subjective sense of danger and threat in the ED (their “threat perception”) and their perceptions of ED clinician-patient communication with subsequent PSS in a sample of patients being evaluated for an acute medical event (suspected ACS). This study was conducted as part of the REactions to Acute Care and Hospitalization (REACH) study, an ongoing observational cohort study of ED predictors of medical and psychological outcomes after evaluation for ACS. We hypothesized that higher degree of threat perception in the ED would be associated with increased PSS in the first week after the ED visit. We also hypothesized that better clinician-patient communication would be associated with decreased PSS, and that clinician-patient communication would be most strongly related to PSS for patients who report the highest levels of perceived threat in the ED.
Methods
Participants and Procedure
English and Spanish speaking participants were enrolled as part of the REACH study during evaluation for ACS from November 2013 to January 2015 at New York-Presbyterian Hospital-Columbia University Medical Center, a single site urban academic medical center ED with 24 hour cardiology and psychiatric services. Patients were identified for the study by a provisional diagnosis of “probable ACS” by the treating ED physician. Exclusion criteria included patients with ST elevations on electrocardiogram in the ED; given the existence of a rapid emergency protocol and transfer to the cardiac catheterization laboratory, enrollment in the ED is not possible for these individuals. Patients were also excluded from participation if they were deemed by the attending physician or research coordinator to be unable to follow the protocol (e.g., due to dementia or substance abuse), in need of immediate psychiatric intervention, or unavailable for follow-up (e.g., due to terminal non-cardiovascular illness).
In the ED, participants completed measures of their ED experience, such as current perceived life threat and vulnerability. In a second interview after transfer to an inpatient bed (or by telephone if inpatient interview was not possible), participants reported on their perception of ED clinician-patient communication and completed an assessment of PSS (median of 3 days after ED presentation, range 0–30 days, 80% within 8 days). Hospital discharge diagnosis was determined by review of the medical record by a research nurse, and was confirmed by a board-certified cardiologist.
All participants provided written informed consent. The study was approved by the Institutional Review Board at the Columbia University Medical Center and conducted in accordance with the Helsinki Declaration as revised in 1989.
Measures
Perceived threat in the ED (Threat Perception)
Participants’ perceptions of life threat and personal vulnerability in response to the suspected ACS event were assessed in the ED using 12 items based on Ozer et al’s[12] meta-analysis of items most predictive of subsequent PTSD. Responses were made on a 4-point Likert scale ranging from “not at all” to “extremely.” These items capture patients’ experience in the ED (e.g., “I am in pain,” “I am afraid,” “I feel helpless,” “I feel vulnerable,” “I worry that I am not in control of my situation”). A total threat score was calculated; responses to these items had good internal consistency (Cronbach’s α=.79). Previous research has utilized similar items to assess perceived vulnerability after acute cardiovascular events.[13]
Clinician-patient communication
Participants’ perceptions of clinician-patient communication was measured with the Interpersonal Processes of Care (IPC) Survey,[14] an 18-item questionnaire that assesses various aspects of interpersonal processes on a 5-point Likert scale from “never” to “always.” Items assess communication style (e.g., “Did the doctor speak too fast?,” “Did the doctor use words hard to understand”), what type of information was conveyed between clinician and patient (e.g., “Did the doctor clearly explain the results of your blood tests?”), and patient-clinician shared decision making (e.g., “Did the doctor involve you with decisions regarding your healthcare/medical treatment?”). A total score reflecting clinician-patient communication quality was constructed, with higher scores reflecting better communication. Aside from excellent psychometrics across a number of studies in multiple countries [15–16], the IPC Survey has been associated with a number of objective indicators/outcomes of communication including length of doctor-patient relationship [17] and pharmacy refill data [18]. Further, it has been found to be sensitive to change in clinician/care delivery behavior [19]. Cronbach’s α for the IPC Survey in this study was .84.
PSS symptoms in response to suspected ACS
The Acute Stress Disorder Scale (ASDS) is a self-report 19-item inventory of early posttraumatic stress symptoms in the acute aftermath of a traumatic event,[20] as the diagnosis of PTSD itself cannot be made within 1 month of a traumatic event. Scores of 50–56 on the ASDS have shown good diagnostic efficiency for predicting PTSD status at 1 month, for example, scores on the ASDS predicted 91% of bushfire survivors who developed PTSD and 93% of those who did not [21] A recent systematic review suggested that the sensitivity of the ASDS is greater than its specificity for long-term PTSD, but high ASDS scores are a strong indicator of risk for PTSD [22] In this study, participants completed the ASDS with reference to the probable ACS event during which the participant was enrolled. A total symptom severity score was calculated by summing responses to the 19 items. Cronbach’s α for the ASDS in the current sample was excellent (α= .90).
Discharge ACS status
REACH enrolls patients who are being evaluated for probable ACS in the ED. However, after all diagnostic tests are completed, many participants receive alternative diagnoses at discharge, such as atrial fibrillation, heart failure exacerbation, or non-cardiac chest pain. A research nurse determined discharge diagnosis from the medical record, and diagnoses were adjudicated by a board-certified cardiologist using the third universal definition of MI [23]. NSTEMI is defined by a episode of presumed ischemic symptoms and a rising and/or falling pattern of serum levels of cardiac biomarkers (preferably troponin) with or without ischemic electrocardiographic changes (ST-segment depression >0.05 mV in two contiguous leads and/or T-wave inversions >0.1 mV in two contiguous leads). UA is defined by presence of ischemic symptoms lasting 20 minutes or longer with recent onset or with an accelerating pattern, or episodes at rest or with minimal effort, and at least one of the following: ischemic electrocardiographic changes (ie, ST-segment depression and/or T-wave abnormalities), an angiogram indicative of coronary artery disease during the current hospital admission, and/or a documented history of coronary artery disease. Non-ACS diagnoses were categorized as: cardiac, non-ACS; non-cardiac, musculoskeletal; non-cardiac, anxiety; non-cardiac, gastrointestinal; non-cardiac, toxic substance; non-cardiac, not otherwise specified; non-cardiac, other (see Table 1).
Table 1.
Sample characteristics.
| Sample characteristics | Mean ± SD |
|---|---|
| Age | 59 ± 12 |
| Women, N (%) | 89 (52) |
| Confirmed ACS diagnosis, N (%) | 158 (33) |
| - Unstable angina, N (% of ACS) | 94 (60) |
| - Non-ST elevation MI, N (% of ACS) | 64 (40) |
| Non-ACS Diagnoses Adjudicated | |
| Cardiac, non-ACS (e.g., hypertensive urgency, atrial fibrillation) | 53 (11) |
| Non-cardiac, musculoskeletal | 62 (13) |
| Non-cardiac, anxiety | 14 (3) |
| Non-cardiac, gastrointestinal | 43 (9) |
| Non-cardiac, toxic substance (e.g., cocaine) | 4 (1) |
| Non-cardiac, not otherwise specified | 122 (26) |
| Non-cardiac, other (e.g., pneumonia, pulmonary embolism) | 18 (4) |
| GRACE score | 91 ± 29 |
| Charlson Comorbidity Index | 2 ± 2 |
| Perceived threat in the EDa | 7.5 ± 5.3 |
| ASDSb | 31 ± 13 |
| IPC Survey clinician-patient communicationc | 71 ± 10 |
Note: ACS: acute coronary syndrome; GRACE: Global Registry of Acute Coronary Events; ASD: Acute Stress Disorder Scale; IPC: Interpersonal Processes of Care; MI: myocardial infarction
Global Registry of Acute Coronary Events (GRACE) risk score
The GRACE index is a post-discharge prediction model for 6-month mortality in patients with cardiac disease derived from a multinational registry.[24] The variables collected from the medical record in the GRACE index are age, history of MI, history of heart failure, presenting pulse rate, systolic blood pressure at presentation, initial serum creatinine level, initial cardiac enzyme levels, ST-segment depression on presenting electrocardiogram, and in-hospital percutaneous coronary intervention. The GRACE index has a range from 1 to 263 points, with higher scores indicating greater mortality risk.
Charlson Comorbidity Index
We abstracted the 19 conditions that are included in the Charlson Comorbidity Index (e.g., congestive heart failure, diabetes mellitus) from the medical record.[25] To calculate the Charlson Comorbidity Index, conditions are weighted from 0 to 6, and points are then summed to generate a total score that can range from 0 to 37. This overall score reflects cumulative increased likelihood of 1-year mortality; the higher the score, the more severe the comorbidity.
Statistical Analysis Plan
Analyses were conducted using the SPSS (IBM SPSS Statistics for Windows, Version 23) and Interaction (Daniel Soper) software packages. We used multiple linear regression to predict PSS (as measured with the ASDS) from threat perceptions during ED evaluation and patient-clinician communication, as well as their interaction. The model adjusted for age, sex, confirmed ACS status, GRACE risk score, and Charlson Comorbidity Index. We tested whether the assumptions of multiple regression were met by evaluating univariate and bivariate normality of modeled variables, variance inflation factor estimates to test for multicollinearity, and examining residuals; all assumptions were met. In sensitivity analyses, we recoded non-ACS diagnoses in two ways to ensure that the nature of the ED diagnosis did not influence perceptions of ED threat or clinician-patient communication, or PSS. First, we adjusted for severity using 2 dummy variables created from grouping ACS, non-ACS cardiac, and non-ACS other diagnoses in 3 groups. Second, we grouped ACS, non-ACS cardiac, toxic substance, and non-ACS other (e.g., pneumonia, pulmonary embolism) into a “potentially life threatening” group and all others into a “non-life threatening” group. Finally, we also conducted analyses with the 4 participants admitted for toxic substances excluded.
Results
Participant Characteristics
Participants were 474 patients admitted to the ED with a provisional diagnosis of ACS. A flowchart of exclusions used to derive the analytic sample is shown in Figure 1. Of those initially deemed eligible for the REACH study by an attending physician, 61% enrolled. Although we do not keep individual records for potential participants who do not consent to participate due to human subjects concerns, we do keep a list of reasons for nonparticipation that arise in order to determine study operations approaches. Reasons for non-participation included severe pain or inability to focus on research questions in the ED, a lack of interest, unavailability for follow-up (e.g., homelessness, imminent travel out of the country), hearing difficulty or no phone for follow-up, rapid transfer or change in medical condition, low fluency in English or Spanish, or a family member objection to participation. The analytic sample for the current study comprised 474 individuals who completed the inpatient interview or telephone assessment.
Figure 1.
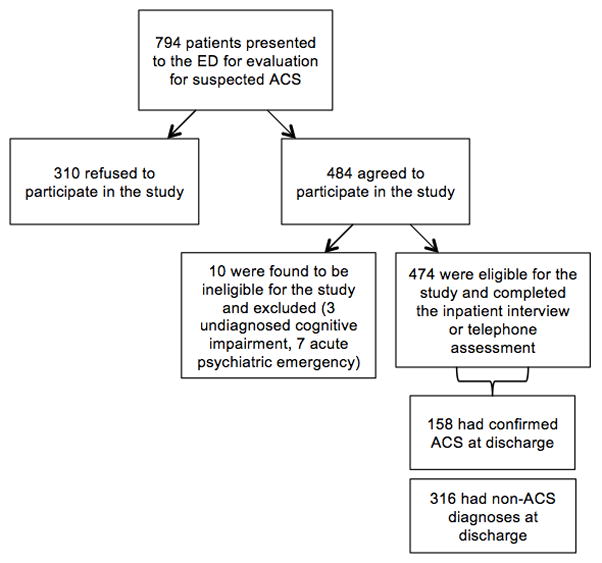
Flowchart of exclusions for deriving the analytic sample.
Participant characteristics are presented in Table 1. One-third (n=158) had confirmed ACS diagnoses at hospital discharge [n=94 (60%) UA, n=64 (40%) NSTEMI]. The remaining participants were given diagnoses such as chest pain without a cardiac diagnosis, another symptom/disease process (e.g., pulmonary embolism, costochondritis), or another cardiac disease (e.g., congestive heart failure exacerbation; see Table 1). There were no missing data from the study. The mean ASDS score was 31 ± 13. Ten percent of the sample screened positive for diagnostic levels of PSS at the least conservative cutoff (ASDS score of 50), and 7% screened positive at the most conservative cutoff (ASDS score of 56).
Association of ED Threat Perceptions and Clinician-Patient Interpersonal Communication in the ED with PSS
Bivariate (Pearson and point biserial) correlations among study variables are given in Table 2. Regression results are given in Table 3. The full model [F(8,473)= 17.28, p<.001; R2 adj = .22] explained 22% of the variance in PSS. The main effects of clinician-patient interpersonal communication (β= −0.11, p= .005) and ED threat perception (β =0.40, p<0.001) were both statistically significant. These main effects were qualified by a statistically significant interaction of ED threat perceptions and clinician-patient communication (β = −0.13, p= .037). As shown in Figure 2, the simple slope for the association of clinician-patient communication with PSS was significantly different from 0 (slope=−0.19, SE slope= .07, p= .002) at the mean of ED threat perceptions, and stronger, though not statistically significant, at 1 SD above the mean of ED threat perceptions (slope=−0.32, SE slope= .32, p= .33). At 1 SD below the mean of ED threat perceptions, there was no association of clinician-patient communication with PSS (slope=−0.06, SE slope= .33, p= .86). In other words, good clinician-patient communication was most strongly associated with lower PSS for patients who perceived moderate to high levels of threat in the ED.
Table 2.
Correlations among study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. ASDS | - | |||||||
| 2. Threat perception | .44 | - | ||||||
| 3. IPC Survey | .16 | −.10 | - | |||||
| 4. Charlson Comorbidity Index | .06 | −.15 | .02 | - | ||||
| 5. ACS confirmed | −.06 | −.02 | .10 | .07 | - | |||
| 6. GRACE score | −.15 | −.18 | .04 | .47 | .04 | - | ||
| 7. Sex | −.06 | −.06 | .03 | .07 | .10 | .03 | - | |
| 8. Age | −.18 | −.17 | .03 | .23 | .07 | .80 | −.07 | - |
Note: ACS: acute coronary syndrome; GRACE: Global Registry of Acute Coronary Events; ASD: acute stress disorder; IPC: Interpersonal Processes of Care. Correlation strengths are designated by shading, with moderate (r = 0.3–0.7) shaded in light gray and strong (r > 0.7) shaded in dark grey.
Table 3.
Multiple regression predicting ASD symptom scores from threat perceptions and quality of clinician-patient communication in the ED
| Variable | B (95% CI) | β | p |
|---|---|---|---|
| IPC Survey score | −0.19 (−0.32, −0.06) | −.12 | <.05 |
| Threat perception | 1.32 (1.04, 1.59) | .39 | <.001 |
| IPC Survey × Threat | −0.03 (−0.06, −0.001) | −.13 | <.04 |
| Age | −0.13 (−0.28, 0.02) | −.12 | .08 |
| Sex | −1.2 (−3.5, 1.0) | −.04 | .29 |
| ACS confirmed | −0.69 (−3.05, 1.67) | −.02 | .56 |
| GRACE score | 0.01 (−0.07, 0.08) | .02 | .20 |
| Charlson Comorbidity Index | 0.17 (−0.44, 0.78) | .03 | .59 |
Note. Model Fit; F(8,473)= 17.28, p<.001; R2 adj = .22; ACS: acute coronary syndrome; GRACE: Global Registry of Acute Coronary Events; ASD: acute stress disorder; IPC: Interpersonal Processes of Care. B=unstandardized regression coefficient. 95% CI= 95% confidence interval. β=standardized regression coefficient.
Figure 2.
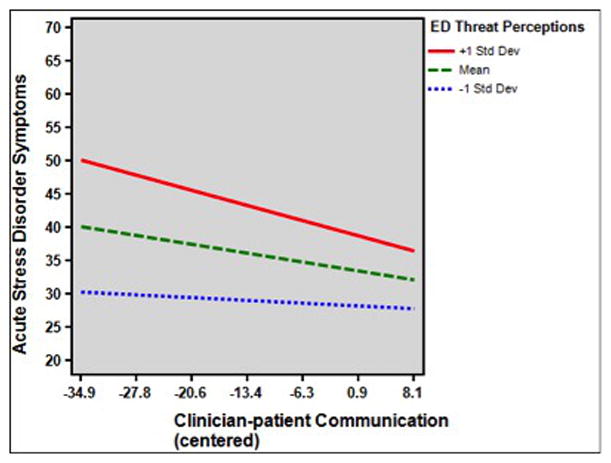
Interaction of clinician-patient communication quality and threat perceptions during emergency department evaluation on subsequent acute stress disorder symptoms.
Sensitivity analyses
In one-way analysis of variance (ANOVA) or t-tests, no ACS grouping variable (e.g., ACS vs. non-ACS cardiac vs. non-ACS other; life threatening vs. non-life threatening) was significantly associated with ED threat, IPC Survey, or ASDS scores (all p’s > .15; no group differed from any other group by more than 2 points on any scale). Further, adjusting for ACS status using these different grouping approaches had no effect on model parameters. Finally, excluding participants determined to have been admitted for chest pain secondary to toxic substances did not influence any finding.
Discussion
Patients being evaluated for a potentially life-threatening ACS in the ED experience a great deal of stress. Indeed, in this study, we found that PSS in the first days after ED evaluation were common, with 7–10% experiencing symptom severity predictive of a subsequent diagnosis of posttraumatic stress disorder. Furthermore, our investigation highlights a modifiable aspect of the ED experience that may help to reduce the likelihood of negative psychological consequences after evaluation for suspected ACS: clinician-patient communication quality. Better scores on clinician-patient communication were associated with lower PSS severity, after adjustment for age, sex, comorbidity, and disease severity. Elevated threat perceptions in the ED were associated with greater severity of PSS as well, and we found that better communication in the ED was most strongly associated with decreased PSS for patients with the highest levels of perceived threat during their ED stay.
PSS following an ACS event have been associated with increased risk for recurrent cardiac events and increased mortality.[26–27] Consistent with previous work across a range of clinical settings, this study suggests that clinician-patient communication in the ED may be protective against the development of PSS. There are multiple implications from these findings. For example, our results offer suggestions for identifying a subset of patients most vulnerable to poor clinician-patient communication who would benefit most from directed efforts to enhance communication. Assessing threat perception in the ED may identify those patients at elevated risk for PSS development, and could prompt ED clinicians to direct special efforts to provide clear and empathic communication to those patients. Previous research suggests that relatively simple interventions to improve clinician-patient communication are effective,[28] although the generalizability of those interventions to ED clinicians is less clear. Additional research is needed to better understand what aspects of clinician-patient communication are most beneficial to patients during their ED stay and how those qualities may best be cultivated. This study may serve as a foundation for that work, as well as offer guidance for intervention development.
Limitations
There were several limitations to our study. Given that clinician-patient communication and PSS were assessed by self-report at the same time point in the study, communication ratings may have been biased by current PSS. However, there was a low correlation (r< .10) between ED threat and communication ratings, which suggests that participants delineated the relatively objective behaviors of physicians from the threatening experience of the ACS. Future work could address this limitation by employing direct observation of clinician-patient interactions during ED stay by research staff.
In our study we assessed patient perception of clinician-patient communication rather than objectively measuring the actual quality of communication between patient and provider. While we attempted to capture many elements of the clinician-patient encounter with our standardized instrument, other aspects of communication not assessed with this measure may also play a role in accounting for the association between clinician-patient communication and PSS after evaluation for suspected ACS. For example, the impact of aspects of the clinician-patient interaction such as perceived empathy were indirectly addressed with some of the questions in the scale, but may have not been captured fully. Furthermore, we were unable to determine whether specific aspects of communication such as information sharing, frequency of contact, or the most positive or negative interaction among different providers in the ED were most important for driving the association with PSS risk. Future work examining clinician-patient interactions may make use of other methodological techniques such as structured interviews or third party observation in an attempt to capture aspects of quality of clinician-patient communication.
Finally, our outcome measure for this study was PSS measured when patients were transferred from the ED to an inpatient bed using the ASDS. While the diagnosis of PTSD cannot be made until after 1 month, the items asked on the ASDS are nearly identical to items asked in PTSD assessments, and past work has found that high scores on the ASDS predict subsequent PTSD development [20]. However, it is important to recognize that rather than specifically targeting PTSD that develops in response to evaluation for suspected ACS, our study identified early posttraumatic stress reactions that are considered risk factors for the development of PTSD.
Conclusions
Posttraumatic stress symptoms following an acute life-threatening medical event may influence patients’ emotional and physical health after their evaluation in the acute setting. Our findings suggest that aspects of the experience of being evaluated for a suspected cardiac event are associated with patients’ subsequent posttraumatic stress reactions. In particular, this research highlights the critical interplay between psychological and interpersonal processes in the ED that may contribute to PSS. Future work should determine whether structured communication interventions or other interpersonal means for reducing threat perceptions can offset risk for PSS in patients evaluated for potentially life-threatening events in the ED.
Acknowledgments
This work was supported by grants HL117832, HL123368, HL128310, and HL130650 from NIH/NHLBI.
Footnotes
Conflicts of interest: None to report.
Author contributions: BC, EC, and DE conceived the study. DE obtained research funding. DE supervised the study and data collection. JS provided statistical advice and drafted the manuscript, and all authors contributed substantially to its revision. BC and DE take responsibility for the paper as a whole.
References
- 1.Gander ML, von Kanel R. Myocardial infarction and posttraumatic stress disorder: frequency, outcome, and atherosclerotic mechanisms. Eur J Cardiovasc Prev Rehabil. 2006;13:165–172. doi: 10.1097/01.hjr.0000214606.60995.46. [DOI] [PubMed] [Google Scholar]
- 2.Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care. 2009;15(2 Suppl):S36–41. [PubMed] [Google Scholar]
- 3.Edmondson D, Kronish IM, Wasson LT, et al. A test of the diathesis-stress model in the emergency department: Who develops PTSD after an acute coronary syndrome? J Psychiatr Res. 2014;53:8–13. [Google Scholar]
- 4.Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ, Kronish IM. Prevalence of PTSD in survivors of stroke and transient ischemic attack: a meta-analytic review. PLOS ONE. 2013;8:e663435. doi: 10.1371/journal.pone.0066435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmondson D, Shimbo D, Ye S, et al. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA Intern med. 2013;173(6):472–475. doi: 10.1001/jamainternmed.2013.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Street RL, Makoul G, Arora NK, et al. How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient educ couns. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Malin JL, Diamant AL, et al. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider–patient communication. Breast cancer res treat. 2013;137(3):829–83. doi: 10.1007/s10549-012-2387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Underhill ML, Kiviniemi MT. The association of perceived provider–patient communication and relationship quality with colorectal cancer screening. Health education behav. 2012;39:555–563. doi: 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris J, Ingham R. Choice of surgery for early breast cancer: psychosocial considerations. Soc sci med. 1988;27(11):1257–1262. doi: 10.1016/0277-9536(88)90355-3. [DOI] [PubMed] [Google Scholar]
- 11.Bredart A, Bouleuc C, Dolbeault S. Doctor-patient communication and satisfaction with care in oncology. Curr opin onc. 2005;17(4):351–354. doi: 10.1097/01.cco.0000167734.26454.30. [DOI] [PubMed] [Google Scholar]
- 12.Ozer E, Best JS, Weiss DS, et al. Predictors of post-traumatic stress disorder in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemar L, Schmid JP, Müller J, et al. Prevalence and predictors of posttraumatic stress disorder in patients with acute myocardial infarction. Heart Lung. 2008;37(2):113–121. doi: 10.1016/j.hrtlng.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Stewart AL, Nápoles-Springer AM, Gregorich SE, et al. Interpersonal Processes of Care Survey: Patient-Reported Measures for Diverse Groups. Health Serv Res. 2007;42(3p1):1235–1256. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lévesque JF, Pineault R, Haggerty JL, et al. Respectfulness from the patient perspective: comparison of primary healthcare evaluation instruments. Healthcare Policy. 2011;7(Special Issue):167–79. [PMC free article] [PubMed] [Google Scholar]
- 16.Beaulieu MD, Haggerty JL, Beaulieu C, et al. Interpersonal communication from the patient perspective: comparison of primary healthcare evaluation instruments. Healthcare Policy. 2011;7(Spec Issue):108. [PMC free article] [PubMed] [Google Scholar]
- 17.PIette JD, Schillinger D, Potter MB, et al. Dimensions of Patient-Provider Communication and Diabetes Self-care in an Ethnically Diverse Population. Journal of Gen Int Med. 2003;18(8):624–633. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratanwongsa N, Karter AJ, Parker MM, et al. Communication and medication refill adherence: the Diabetes Study of Northern California. JAMA Internal Med. 2013;173(3):210–218. doi: 10.1001/jamainternmed.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schillinger D, Handley W, Wang F, et al. Effects of Self-Management Support on Structure, Process and Outcomes Among Vulnerable Paitents with Diabetes: A three-arm practical clinical trial. Diabetes Care. 2009;32(4):559–566. doi: 10.2337/dc08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant RA, Moulds ML, Guthrie RM. Acute Stress Disorder Scale: a self-report measure of acute stress disorder. Psychol Assess. 2000;12(1):61. [PubMed] [Google Scholar]
- 21.Bryant RA, Creamer M, O’Donnell ML, et al. A multisite study of the capacity of acute stress disorder diagnosis to predict posttraumatic stress disorder. J Clin Psychiatry. 2008;69(6):923–929. doi: 10.4088/jcp.v69n0606. [DOI] [PubMed] [Google Scholar]
- 22.Bryant RA. Acute Stress Disorder as a Predictor of Posttraumantic Stress Disorder: A Systematic Review. Journal of Clinical Psychiatry. 2011;73(2):233–239. doi: 10.4088/JCP.09r05072blu. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen Kristian, Alpert Joseph S, Jaffe Allan S, White Harvey D, Simoons Maarten L, Chaitman Bernard R, Katus Hugo A, et al. Third universal definition of myocardial infarction. JACC. 2012;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. Jama. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 25.Charlson M, Szatrowski TP, Peterson J, et al. (1994). Validation of a combined comorbidity index. J clin epi. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 26.Edmondson D, Rieckmann N, Shaffer JA, et al. Posttraumatic stress due to an acute coronary syndrome increases risk of 42-month major adverse cardiac events and all-cause mortality. J psych res. 2011;45(12):1621–1626. doi: 10.1016/j.jpsychires.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shemesh E, Yehuda R, Milo O, et al. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med. 2004;66:521–526. doi: 10.1097/01.psy.0000126199.05189.86. [DOI] [PubMed] [Google Scholar]
- 28.Hobma S, Ram P, Muijtjens A, et al. Effective improvement of doctor–patient communication: a randomised controlled trial. Br J gen pract. 2006;56(529):580–586. [PMC free article] [PubMed] [Google Scholar]


