Abstract
Atherosclerosis is a chronic inflammatory condition. Many pro-inflammatory factors including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), and adhesion molecules including intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) are expressed in atherosclerotic lesions. The plants of genus Vaccinium are rich in anthocyanins with anti-inflammatory effects. This study aimed to evaluate the effects of Vaccinium arctostaphylos fruit extract on the serum level of TNF-α, IL-6, ICAM-1, and VCAM-1 in adult patients with mild hyperlipidemia to detect its possible inhibitory effects on progression of atherosclerosis. In a randomized double-blind placebo-controlled clinical trial, eligible hyperlipidemic patients were randomly and equally divided in to two groups of study drug or placebo control to receive either the Vaccinium extract or placebo capsules, respectively, twice daily for four consecutive weeks. Each drug capsule contained 0.8 mg of anthocyanins. Serum levels of TNF-α, IL-6, ICAM-1, and VCAM-1 were measured before and after the interventions and finally were compared.A total of 8 men and 12 women in drug group as well as 11 men and 9 women in placebo group completed the study (P = 0.527). The use of Vaccinium extract significantly reduced only the IL-6 level (P = 0.037); however, this reduction was not significant compared to placebo (P = 0.062). Consumption of Vaccinium arctostaphylos fruit extract with the dose of 500 mg twice daily did not show any significant effect on serum levels of TNF-α, IL-6, ICAM-1, and VCAM-1 in adult hyperlipidemic patients. However, considering slight decrease in the level of IL-6, ICAM-1, and VCAM-1, the use of higher doses with longer duration might have significant effects on these factors.
Keywords: Clinical trial, Hyperlipidemia, Inflammatory, factors, Vaccinium arctostaphylos
INTRODUCTION
Inflammation plays a critical role in the development and progression of athero-sclerosis (1). The cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF–α) released by stimulated T lymphocytes, affect the macrophages/monocytes to elaborate mediators of innate immunity such as interleukin-1 (IL-1) and IL-6 in response, as well as transforming growth factor-α (TGF-α). These cytokines in turn mediate paracrine signaling to endothelial and vascular smooth muscle cells (2). On the other hand, products of oxidized lipoproteins and critical factors in the atherogenesis, could provoke vascular wall cells to produce cytokines (1,3,4). Thus, an early stimulus for the recruitment of inflammatory cells to the lesion might arise from the production of cytokines by local vascular wall cells that elicit expression of adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) as well as chemoattractants (1).
The adhesion molecules, expressed by cytokine–stimulated endothelial cells, bind to monocytes and T lymphocytes; subsequently, these inflammatory cells accumulate in the early atherosclerotic plaque (5,6). In the arterial intima, monocytes mature into macrophages. In the plaque, these mono-nuclear phagocytes trap modified lipoproteins contributing to formation of foam cells (1) and fatty streaks, the anatomical hallmark of early atherosclerosis (7,8).
Due to contribution of inflammation to the development of atherosclerotic plaques, anti-inflammatory drugs that are aimed at reducing the progression of atherosclerosis and risk of cardiovascular events are now in development. Such an effect has been shown for HMG-CoA reductase inhibitors (statins) (9).
Vaccinium arctostaphylos L. (Caucasian Whortleberry), a plant found in northern forests of Iran and commonly known as “Qare-Qat”, has fruits (berries) rich in anthocyanins (10). Anthocyanins are compounds with antioxidant, anti-inflammatory, anti-athero-sclerotic, and anti-hyperlipidemic activities (11,12,13,14,15). This study aimed to evaluate the effects of fruit extract of this plant on serum levels of IL-6, TNF-α, ICAM-1, and VCAM-1 in hyperlipidemic adult patients to assess its potential protective effect against atherosclerosis.
MATERIALS AND METHODS
Plant material and extraction
Fresh ripe berries of V. arctostaphylos were collected from the forests of Asalem, Iran, in August 2013 and were identified by department of pharmacognosy, faculty of Pharmacy, Isfahan University of Medical Sciences. After drying at room temperature (20–22°C), the berries were extracted by maceration with ethanol 70% (Stalk, Iran).
The extract was then filtrated, concentrated under vacuum using rotary evaporator (Heidolph, Germany), dried by a freeze drier (Alpha 2-4 LD plus, Japan) and standardized by spectrophotometric determination of anthocyanin content.
Extract standardization
The obtained extract was standardized based on the total anthocyanin content using the pH differential method (16). For this, two 1-g dried extract samples were dissolved in 10 mL of buffer solution (pH, 1) composed of 125 mL of KCl 0.2 M (Merck, Germany), 375 mL of HCl 0.2 M (Merck, Germany), and 10 mL of buffer solution with pH of 4.5 composed of 400 mL sodium acetate 1 M (Merck, Germany), 240 mL of HCl 1 M, and 360 mL of water. Both solutions were diluted 10 times with the same buffer and their absorbance was read at 510 nm using a spectrophotometer (PerkinElmer, USA). Total anthocyanin content was determined by the following equation:
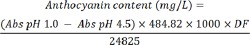
In the above equation, 484.82 is the molecular mass of cyanidin-3-glucoside chloride, 24825 is molar absorptivity at 510 nm at pH = 1, and DF is the dilution factor that is 100.
Based on the above method, the anthocyanin content was 1.6 mg/g of the prepared powder.
Preparation of drug and placebo capsules
Each drug capsule was filled with 500 mg of dried extract powder equivalent to 0.8 mg of total anthocyanins. Each placebo capsule was filled with 500 mg of dried granulated powder of tribasic calcium phosphate. The drug and placebo capsules were similar regarding shape, color, and size.
Patient selection
The inclusion criteria for participation of patients in the study were: (a) age ≥ 18 years, (b) serum lipid levels: total cholesterol 200–239 mg/dL and / or triglyceride (TG) 150–199 mg/dL and / or LDL-C 130–160 mg/dL, (c) non-smoking, (d) free of diseases affecting serum lipids or inflammatory markers (e.g. diabetes mellitus, thyroid disorders, nephrotic syndrome, rheumatologic disorders, and any infection), (e) not using drugs or supplements affecting serum lipids (e.g. statins, fibrate derivatives, estrogens, progestins, beta-blockers, thiazide diuretics, and fish oil) within the last 3 months, (f) free of liver disease (serum level of alanine aminotransferase (ALT) > 2 times upper limit of normal), (g) free of kidney disease (serum level of creatinine > 1.2 mg/dL), and (h) not being pregnant or lactating (for women).
The exclusion criteria included: (a) irregular use of the capsules (use of less than 80% of capsules during the study), and (b) allergic reaction to Vaccinium extract.
Study design and interventions
This was a randomized, double-blind, placebo-controlled clinical trial conducted in Isfahan Cardiovascular Research Center affiliated to Isfahan University of Medical Sciences, Isfahan, Iran, from November 2013 to August 2014. The study was registered in Iran Registry of Clinical Trials (IRCT) with the record number of IRCT201507309662N10. Informed consent was obtained from all participants and the study protocol was approved by the ethical committee of Isfahan University of Medical Sciences. Patients who met the inclusion criteria were randomly and equally assigned to either the study drug (V. arctostaphylos extract) or placebo groups. The demographic characteristics were recorded for all patients. Prior to any intervention, 5 mL of blood sample was obtained from each subject, centrifuged at 2000 rpm for 10 min, and the separated serum was frozen at -70°C. At appropriate time, the serum levels of IL-6, TNF-α, ICAM-1, and VCAM-1 were determined using specific ELISA Kits (Boster Biological, Fremont, CA, USA). Also, for detection of any possible renal and hepatic adverse effect of the extract, the serum levels of ALT, aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine were measured using related assay kits (Pars Azmoon, Iran). The patients of drug and placebo groups were instructed to use drug or placebo capsule (500 mg), respectively, twice daily with food for four weeks. All patients were advised to maintain their habitual diet and physical activity and report any adverse effect during the study. The patients’ compliance was evaluated by counting their capsules at the end of use and their results were applied for data analysis if they used more than 80% of their capsules. At the end of four weeks, all mentioned parameters were again determined and compared with baseline values as well as between groups. For randomization and blindness, each capsule container was given a code according to the type of its content (drug or placebo). When giving a container to each patient, its code was recorded on his / her own consent form. At the end of the intervention and after determination of the patient's own results, the recorded code was identified in terms of the type of intervention. All participants, the physician, and the laboratory personnel were blind to the intervention type.
Statistical Analysis
SPSS 20.0 software (SPSS Inc., Chicago, USA) was used for statistical analysis of obtained data. Kolmogorov-Smirnov test was performed to assess distribution pattern of continuous data. Baseline values were compared using independent-samples t-test and Mann-Withney U test. For comparison of pre- and post-intervention values within each group, paired-samples t-test and Wilcoxon singned-rank test were performed for normally and non-normally distributed variables, respectively. Analysis of covariance (ANCOVA) was used for comparing parameters between drug and placebo groups with statistical control of baseline values. Chi-square test was done for comparison of gender distribution in two groups. P < 0.05 was considered as significance level.
RESULTS
Over the study period, a total of 65 hyperlipidemic subjects were screened for eligibility, of whom 46 patients met inclusion criteria and participated in the research after equal and random assignment in two groups. During the trial, three subjects were excluded from each group due to either irregular use of capsules (use of less than 80% of the capsules) or altering their usual diet. So, a total of 20 subjects in each group completed the study (Fig. 1).
Fig. 1.
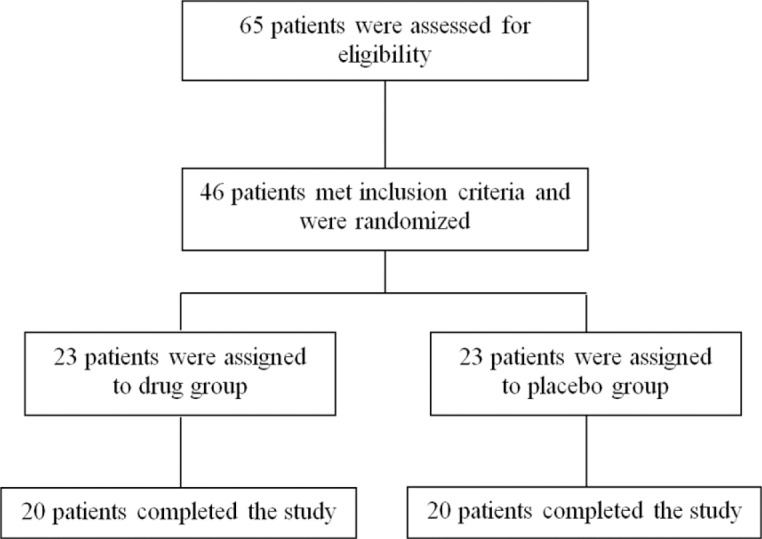
Flowchart of patients’ enrollment in the study
According to the normality test of collected data, all evaluated variables had normal distribution, except for VCAM-1 values and pre-intervention values of ICAM-1 and TNF-α in placebo group as well as post-intervention values in drug group necessitating use of non-parametric statistical tests for these variables.
Table 1 shows baseline demographic and clinical characteristics of study subjects. As shown, the patients of two groups were matched in terms of all baseline parameters including serum lipids except for TNF-α.
Table 1.
Baseline demographic and clinical characteristics of study subjects. The values are presentedas mean (SD).
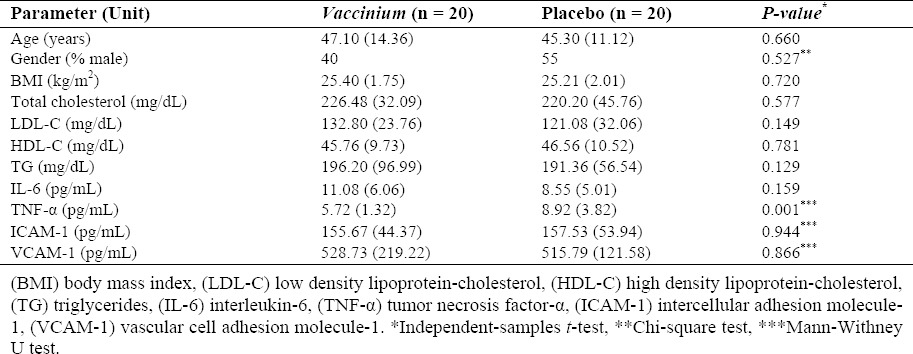
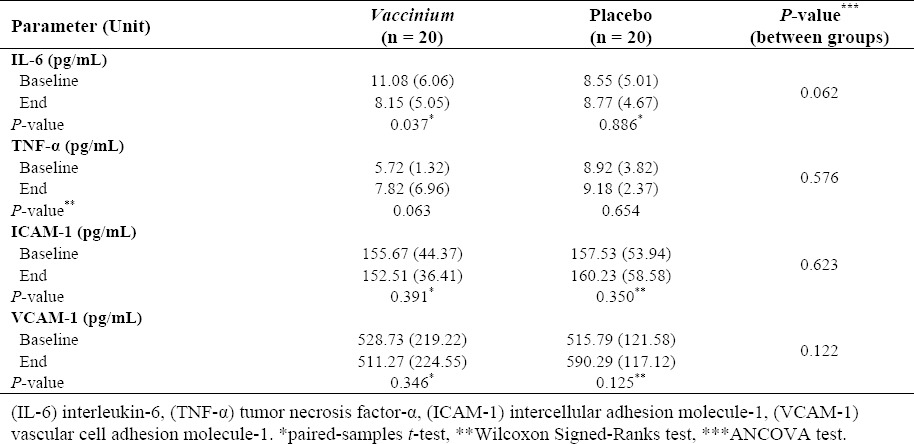
Table 2 shows the effects of interventions on evaluated variables after four weeks in the study subjects. As shown, V. arctostaphylos fruit extract reduced the serum level of IL-6 (P = 0.037); however, this effect was not statistically significant compared to placebo (P = 0.062). All other parameters were not affected significantly by the interventions.
Table 2.
The effects of interventions on tested parameters after four weeks in the study subjects. The values are presented as mean (SD).
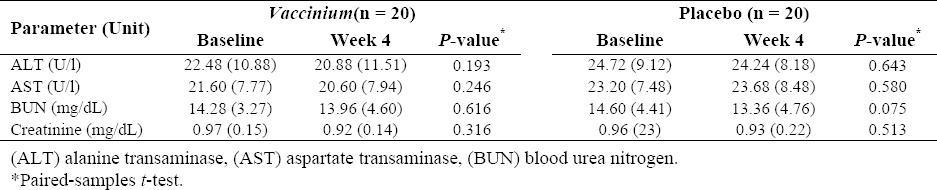
As shown in Table 3, the interventions did not affect the kidney and liver function tests. Furthermore, during the study, four patients from drug group complained of gastrointestinal upset due to consumption of capsules on an empty stomach. No other side effects were reported by any patients.
Table 3.
The effects of interventions on the liver and kidney function tests of the study subjects after four weeks. The values are presented as mean (SD).
DISCUSSION
Our study showed that the use of fruit extract of V. arctostaphylos has no substantial effect in reduction of inflammatory factors in adult hyperlipidemic subjects. At the best of our knowledge, this is the first clinical study on this plant evaluating such an effect. However, several experimental and clinical studies have evaluated anti-inflammatory effects of other species of the genus Vaccinium in various groups of subjects. In a placebo-controlled study conducted by Kolehmainen et al., daily consumption of 400 g of fresh bilberries of V. myrtillus by patients with metabolic syndrome reduced their serum levels of CRP, IL-6, and IL-12 (17). In the clinical study of Karlsen et al., the effects of bilberry juice at 330 mL/day on plasma levels of inflammatory markers in subjects at increased risk of cardiovascular disease (CVD) were assessed (18). Based on the results, bilberry significantly reduced the plasma levels of CRP, IL-6, and IL-15, while the TNF-α level increased. Therefore, these results are similar to ours regarding the effects on IL-6 and TNF-α levels. In another clinical trial performed by Ruel et al. on healthy sedentary men, the consumption of increasing daily doses of cranberry juice cocktail (125, 250, and 500 mL/day) over three successive periods of 4 weeks resulted in reduction of serum levels of ICAM-1, VCAM-1, and OxLDL (19). According to the authors, these effects were attributed to polyphenolic compounds of the cranberry. On the other hand, in the study of Basu et al., daily use of 50 g freeze-dried blueberries by metabolic syndrome patients for 8 weeks showed no significant effects on serum CRP, ICAM-1, VCAM-1, and IL-6 (20). The various amounts of consumed doses as well as different applied preparations, study population, and intervention durations might have contributed to different results of these studies. Considering slight insignificant reduction of IL-6, ICAM-1, and VCAM-1 by V. arctostaphylos extract in our study, it seems that the use of higher doses for longer durations might have more significant effects on these biomarkers.
CONCLUSION
In conclusion, consumption of V. arctostaphylos fruit extract with daily dose of 1000 mg did not show any significant effect on serum levels of IL-6, TNF-α, ICAM-1, and VCAM-1 in adult hyperlipidemic patients. Therefore, it cannot be considered as a protective supplement against atherosclerosis progression. However, more studies for evaluation of higher doses of the extract for longer durations are recommended.
ACKNOWLEDGEMENTS
This study was financially supported by the Vice-Chancellery for Research and Technology at the Isfahan University of Medical Sciences. The authors would like to acknowledge MrsKeshvari and the staff of Laboratory Department of the Isfahan Cardiovascular Research Institute for their assistance.
REFERENCES
- 1.Libby P. Inflammation in Atherosclerosis. ArteriosclerThrombVascBiol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Salomon RN, Payne DD, Schoen FJ, Pober JS. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989;21(4):3677–3684. [PubMed] [Google Scholar]
- 3.Lipton BA, Parthasarathy S, Ord VA, Clinton SK, Libby P, Rosenfeld ME. Components of the protein fraction of oxidized low density lipoprotein stimulate interleukin-1 alpha production by rabbit arterial macrophage-derived foam cells. J Lipid Res. 1995;36:2232–2242. [PubMed] [Google Scholar]
- 4.Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kübler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. ArteriosclerThrombVasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 5.Aqel NM, Ball RY, Waldmann H, Mitchinson MJ. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- 6.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. ArteriosclerThrombVascBiol. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011;10(5):365–376. doi: 10.1038/nrd3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickavar B, Amin G. Anthocyanins from Vaccinium arctostaphylos berries. Pharm Biol. 2004;42([4]-[5]):289–291. [Google Scholar]
- 11.Tsuda T, Horio F, Kitoh J, Osawa T. Protective effects of dietary cyaniding 3-o-β-d-glucoside on liver ischemia-reperfusion injury in rats. Arch Biochem Physiol. 1999;368(2):361–366. doi: 10.1006/abbi.1999.1311. [DOI] [PubMed] [Google Scholar]
- 12.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 13.Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44:217–222. doi: 10.1161/01.HYP.0000135868.38343.c6. [DOI] [PubMed] [Google Scholar]
- 14.Ling WH, Cheng QX, Ma J, Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J Nutr. 2001;131(5):1421–1426. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- 15.Xia M, Ling WH, Ma J, Kitts DD, Zawistowski J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein e deficient mice. J Nutr. 2003;133(3):744–751. doi: 10.1093/jn/133.3.744. [DOI] [PubMed] [Google Scholar]
- 16.Hasanloo T, Sepehrifar R, Hajimehdipoor H. Levels of phenolic compounds and their effects on antioxidant capacity of wild Vaccinium arctostaphylos L. (Qare-Qat) collected from different regions of Iran. Turk J Biol. 2011;35:371–377. [Google Scholar]
- 17.Kolehmainen M, Mykkänen O, Kirjavainen PV, Leppänen T, Moilanen E, Adriaens M, et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. MolNutr Food Res. 2012;56(10):1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 18.Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, et al. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49(6):345–355. doi: 10.1007/s00394-010-0092-0. [DOI] [PubMed] [Google Scholar]
- 19.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br J Nutr. 2008;99(2):352–359. doi: 10.1017/S0007114507811986. [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]


