Abstract
Carbohydrate intake, preference, and taste thresholds may be altered in current and former cigarette smokers, which may mediate weight gain and risk for obesity in individuals who quit smoking. Attempts to model these effects in rodents have primarily used noncontingent nicotine administration. The purpose of this research was to characterize changes in chow and sucrose intake in rats during a 23-h access model of i.v. nicotine self-administration (NSA), in which rats lever-pressed for chow, sucrose, and nicotine under concurrent fixed-ratio (FR) 1 schedules. Male rats were assigned to one of three groups that differed in food and drug availability. The Nicotine C+S group had concurrent access to nicotine, chow, and sucrose. The Saline C+S group had access to saline, chow, and sucrose. The Nicotine C-Only group had access to nicotine and chow, but not sucrose. Changes in food intake and weight gain were assessed during baseline, NSA, and nicotine withdrawal (i.e., saline extinction). Weight gain was significantly slowed during NSA and increased during withdrawal, but did not differ between the nicotine groups. NSA produced a significant decrease in both chow and sucrose intake. Gradual tolerance to nicotine’s effects on sucrose, but not chow intake, occurred. During withdrawal, chow and sucrose intake increased, with a larger percent increase in sucrose intake compared to chow. The proportion of total food intake from sucrose was greater at the end of withdrawal compared to baseline, indicating a history of nicotine intake changed dietary preference. Combined, these results indicate that sucrose intake is more resistant to nicotine’s appetite suppressant effects and withdrawal from nicotine produces a greater increase in sweet food intake alongside general increases in chow intake. Changes in overall food intake in current and ex-smokers may lead to increased risk for obesity and other health problems, potentially limiting the benefit of quitting smoking.
Keywords: Nicotine self-administration, Food intake, Sucrose, Body weight, Concurrent access, Multiple reinforcers
Introduction
The two leading causes of preventable death in the United States are smoking [1] and obesity [2]. Within the last decade, the aggregate national cost of overweight/obesity and smoking have been estimated at up to $209 billion [3,4] and $193 billion [1], respectively. In the last 40+ years, overweight and obesity have increased alongside a decrease in smoking prevalence in the United States [5] and other countries[6,7], with up to a quarter of this increase attributable to smoking cessation [8]. Ex-smokers gain at least 4–5 kg in the first year after quitting [9], which is associated with increased mortality, incidence of hypertension, and impaired glucose tolerance [10]. This increase in disease risk is further amplified in 13–33% of individuals who gain weight above this average (>15kg; [8,11,12])
Nicotine is considered the principle component in cigarettes responsible for smoking’s effects on body weight [13,14]. Nicotine exposure via smoking in humans or direct administration in animals results in weight loss, whereas smoking cessation in humans and withdrawal from nicotine in animals causes weight gain [15–17]. While nicotine may affect body weight by influencing any of the three factors involved in energy balance (i.e., food intake, metabolism, and physical activity), investigations into nicotine’s effects on physical activity and metabolism have been somewhat inconclusive [18–25]. In contrast, nicotine’s effects on food intake are clearer [15–17], and estimates suggest that up to 70% of smoking’s effects on body weight are caused by reductions in food intake [13,14].
Nicotine’s effects on energy intake may be largely facilitated by the altered consumption of specific types of foods, particularly those high in carbohydrates. Studies of changes in carbohydrate intake suggest that quitting smoking is associated with increased caloric intake in the form of high sugar and high fat foods [26–28]. Furthermore, post-cessation weight gain is correlated with total carbohydrate intake [27], which appears to drive the increase in food intake observed within the first few days of abstinence [29]. Withdrawal from nicotine may also produce an enhanced motivation for carbohydrates. For example, females who abstain from smoking for 24 hours work harder to obtain carbohydrate snacks, even when the probability of earning those snacks is decreased [29,30]. The degree to which such fluctuations in carbohydrate intake are mediated by specific changes in taste preferences for carbohydrates is less conclusive. There have been several reports of current smokers showing increased sucrose preferences [31,32], while other studies have shown that current smokers have a reduced preference for sweet solutions [33]. Examinations of sucrose preference ratings during withdrawal have been limited, with one investigation showing that quitting smoking increases pleasantness ratings for sucrose solutions [27], while a separate study failed to show a change in sucrose preferences [34].
Animal models can be useful for clarifying the role of nicotine in smoking’s effects on body weight and food intake in several ways. First, they can isolate the direct effects of nicotine on ingestive behaviors from other tobacco constituents. Second, they allow for the study of central nervous system mechanisms underlying nicotine’s effects on food intake independent of the potential effects of smoke exposure on peripheral gustatory and olfactory receptor function and taste thresholds. Third, they allow for the preclinical evaluation of novel pharmacotherapies for smoking cessation and weight control.
Unfortunately, animal models have not consistently replicated the specific changes in food intake and body weights observed in human smokers and have differed between studies depending on the route of administration used. Nicotine administration in animal models has occurred primarily through continuous subcutaneous infusions [15,35] or multiple systemic injections [17,36]. Only transient decreases in food intake over time have been seen during continuous [37] and intermittent nicotine administration [19,38,39], with decreases in body weight throughout the administration period. Increases in food intake and body weight have not been observed following withdrawal from i.p. nicotine injections [19,39], whereas withdrawal from continuous nicotine delivery has produced increases in food intake above control levels and increases in weight gain back to control levels [17,37]. These studies contrast with the human research suggesting that weight gain is greatest in the first three months after quitting smoking [9] and that ex-smokers weigh more than nonsmokers [16].
Such discrepancies between the human and animal literature may be due to differences in the level and pattern of nicotine exposure. These routes of administration fail to emulate the episodic changes in plasma nicotine levels associated with cigarette smoking [40–43] producing fewer and larger spikes (multiple injections) or no fluctuations (continuous delivery) in plasma nicotine levels [44], compared to smoking. In contrast to multiple injections and continuous infusions, intraveous (i.v.) administration of nicotine can produce a pattern of plasma nicotine concentrations that are more consistent with that of smoke inhalation, with persistent decreases in weight gain and food intake during nicotine administration and increases during withdrawal [45,46]. However, in Grebenstein et al. 2013, withdrawal-mediated increases in weight gain and food intake following noncontingent i.v. nicotine administration were delayed, despite evidence in smokers that suggests increases in appetite occur within days of a quit attempt [30,47].
Although considerable human research has examined the selective effects of smoking on the intake of different types of foods, animal research on this issue is limited. To our knowledge, Grunberg and colleagues conducted the only studies examining the effects of nicotine on the intake of multiple types of concurrently available foods. In these studies, males showed a significant reduction in consumption of a sweetened solution, without any change in chow intake during nicotine administration [48] and a slight reduction in sweet low calorie food following high doses of nicotine delivered via minipumps [35]. Given the aforementioned limitations of continuous infusions, the purpose of the present study was to evaluate the generality of the findings of Grundberg and colleagues by examining the effects of nicotine self-administration on the intake of different concurrently available foods to better model the nature of nicotine exposure associated with smoking. A 23-h extended access model of i.v. nicotine self-administration (NSA) was used, with chow pellets, sucrose pellets, and nicotine all made concurrently available. In addition to its similarity to nicotine exposure in human smokers, the i.v. NSA model was also selected for other reasons. First, it allows animals to consume food and nicotine together, without any experimenter-imposed regulations on food or drug availability. Thus, as in smokers, the effects of nicotine on food intake are a function of what’s been self-administered by (i.e., is reinforcing to) the animal rather than an arbitrary dosing regimen that may have aversive or toxic side effects. Second, concurrent availability allows nicotine to serve as a competing, alternative reinforcer, thereby modeling the economic context of drug and food intake. Third, NSA produces a disruption of the circadian regulation of food intake [46], which is unaccounted for in noncontingent studies where nicotine is administered through continuous infusions or multiple i.p. injections. Third, NSA eliminates the potential confounds of stress effects of noncontingent administration on food intake (see [49]).
Material and methods
2.1. Animals
Twenty-five male Holtzman rats (Harlan, Indianapolis, IN) weighing 300–350 g at arrival were used in this study. Upon arrival, all rats were individually housed in a temperature- and humidity- controlled colony room with unlimited access to food and water under a reversed 12-h light/dark cycle (lights off at 11:00 h) for approximately one week. Rats were then moved to operant conditioning chambers in a separate room under the same light/dark cycle following recovery from catheter implantation for NSA (see section 2.4). Concurrent access to chow pellets, sucrose pellets, and water was available within the operant chambers on a fixed-ratio 1 (FR1) schedule. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2013 NIH guide for the Care and Use of Mammals in Neuroscience and Behavioral Research.
2.2. Apparatus
Each operant conditioning chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, an aluminum ceiling, and a stainless steel grid floor. Two standard response levers (ENV-110RM, Med Associates) were located on the front wall 7 cm above the chamber floor and a third response lever was located on the back wall 7 cm above the chamber floor, for the delivery of nicotine. Standard grain pellets: 45 mg Rodent Dustless Precision Pellets (formula PJAI, TestDiet, Richmond, IN, USA; total Kcal/g: 3.303; breakdown: 0.796 protein, 0.345 fat, and 2.162 carbohydrate) were dispensed via a feeder (ENV-203M-45, Med Associates) into a food receptacle on the front wall located between two levers. Sucrose pellets: 45 mg Rodent Dustless Precision Pellets (formula PJFSC, TestDiet, Richmond, IN, USA; 45g; total Kcal/g: 3.404; breakdown: 3.404 carbohydrate) were dispensed via a feeder into a food receptacle on the back wall between a water bottle and another lever. Stimulus lights were located 2 cm above all three levers. At the start of the experiment, the nicotine lever was not present. Water was continuously available via a spout mounted on the back wall of the chamber, to the left of the food receptacle. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise, and was equipped with a fluorescent light that provided ambient illumination during the light-on phase of the light cycle. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside each cubicle delivered infusions through tygon tubing connected to a fluid swivel mounted above the chamber, and from the swivel through the spring leash connected to a guide cannula mounted in a harness assembly on the back of the rat. Med-PC IV (Med Associated, St Albans, VT) software was used for operating the apparatus and recording data.
2.3. Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved into sterile saline. The pH of the solution was adjusted to 7.4 with dilute NaOH, and heparin (30 units/ml) was added to help maintain catheter patency. Nicotine doses are expressed as the base. Methohexital (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline (0.1ml, 10 mg/ml, i.v.) and used to assess catheter patency.
2.4. Surgery
Each rat was implanted with a chronic indwelling catheter into the right jugular or femoral vein under ketamine (100.00 mg/kg, i.m.)/dexmedetomidine (0.25 mg/kg, i.m.) anesthesia, with atipamezole (1.00 mg/kg, s.c.) for reversal following surgery. The catheter was externalized between the scapulae and attached to a vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least four days after surgery, during which time they received daily i.v. infusions of ceftriaxone (5.00 mg/day) and buprenorphine (0.10 mg/kg, s.c.; first 2 days only) for analgesia. When necessary, catheter patency was verified using methohexital (1.50 mg, i.v.). Catheter occlusion, as seen by failure to exhibit signs of anesthesia, resulted in the implantation of a new catheter in the left femoral vein or exclusion from the study if the second catheter failed. The total number of animals that received a femoral implant during the course of the investigation was 4, out of 25.
2.5. Food intake and drug self-administration procedure
The experimental protocol was divided into three phases that each lasted at least 10 days and until behavior stabilized (see below). Sessions were 23-h/day, and body weights were measured daily during the final 1 hour period of the light cycle, when housekeeping duties were performed. During the baseline phase, standard grain pellets (hereafter referred to as ‘chow’ pellets) and sucrose pellets were each available under an FR1 schedule of reinforcement on separate levers (described in section 2.2). Each lever press for chow and sucrose was accompanied by a 1 second presentation of a white cue light directly above the food lever(s). Heparinized saline (see section 2.3) was administered daily via programmed infusions (30/inf/day) during baseline to maintain catheter patency. Total food intake, chow intake, and sucrose intake were measured daily. Following stable chow and sucrose intake, defined as <20% coefficient of variance over five consecutive days without any trends, the treatment phase began. Stability criteria for food intake were determined based on the observed variability in sucrose intake across days, particularly for those animals that consumed less than 100 pellets per day of sucrose. At the start of the treatment phase, the nicotine lever was introduced into the chambers and nicotine was available under an FR1 schedule of reinforcement. Following each lever press, the stimulus light above the nicotine lever was illuminated for 7 seconds and nicotine (0.06 mg/kg/inf) was infused in a volume of 100 μl/kg at a rate of 50 μl/s. This training dose was chosen because it is readily self-administered and has been shown in previous studies to produce reliable decreases in weight gain and food intake [45,46]. Acquisition of NSA was defined as a minimum of 10 infusions per day, and was considered stable when the coefficient of variance was less than 15% and there were no obvious trends over five consecutive days. Following stable NSA, the extinction phase began, and nicotine was replaced with saline. Stable extinction of NSA was defined as a >50% decrease in infusions per day and no visible trends over five consecutive days.
Prior to the start of the experiment, rats were randomly assigned to one of three groups that differed in food and drug availability. The Nicotine C+S group (n = 9) had concurrent access to chow, sucrose, and nicotine during the treatment phase (described above). The Saline C+S group (n = 8) had concurrent access to chow, sucrose, and saline. To control for changes in food intake and weight gain over time, each Saline C+S control animal was yoked to a Nicotine C+S rat, such that when a Nicotine C+S rat achieved stability and moved on to the next phase, so did its yoked Saline C+S rat. The final group, Nicotine C-Only, had concurrent access to chow only and nicotine. This group was included as a control for the potential for sucrose availability to decrease drug self-administration and therefore attenuate the expected decrease in body weight and food intake. Responses on the third (inactive) lever for this group were recorded but had no programmed consequences. Stability was defined as at least 10 infusions, with a coefficient of variance <20%, no visible trends, and a 2:1 active:inactive lever response rate.
2.6. Data Analysis
2.6.1. Nicotine self-administration
The mean number of infusions during the first 10 days and the last day of the treatment phase were analyzed using a two-way ANOVA, followed by Holm-Sidak multiple comparisons tests to analyze differences between the three groups on each day. The same approach was used to analyze changes in the mean number of infusions between the three groups during the extinction phase.
2.6.2. Body weight
Stable body weight was recorded over the final five consecutive days of baseline. Changes in body weight during the final four days of baseline were expressed as a percent change of body weight on the first stable baseline day to measure the rate of weight gain at baseline. During the treatment phase, daily changes in body weight over the first 10 days were calculated relative to the last five stable days of the baseline phase and plotted as percent change in body weight (g) over time. Body weight change during the extinction phase was calculated similarly, with body weight during the first 10 days of extinction expressed as a percent change of the final five stable days of the treatment phase. Linear regression analyses were used to determine if the rate of weight gain (i.e., slope) between the three groups differed. A two-way ANOVA was then used to compare the percent change in body weight between groups, followed by Holm-Sidak post-hoc tests with each phase analyzed separately, to compare groups at each of the first 10 days of baseline, treatment, and extinction.
2.6.3. Daily time course of absolute chow and sucrose intake
Mean absolute chow intake over the final five days of baseline and the first 10 days of each phase was analyzed using two-way ANOVAs. Post-hoc tests with Dunnett’s corrections for multiple comparisons were used for daily within group comparisons of chow intake relative to the mean of the final five days of the previous phase. Post-hoc tests with Holm-Sidak corrections for multiple comparisons were used to determine differences between all three groups. Absolute sucrose intake was analyzed similarly.
2.6.5. Total food intake at stability during each phase
The mean total food intake across the last five sessions of each phase (i.e., stability) was compared between the Nicotine C+S, Nicotine C-Only, and Saline C+S groups, using a two-way ANOVA, with phase and group as factors. Holm-Sidak post-hoc tests were used to analyze differences in total food intake between the three groups at each phase and Dunnet’s post-hoc tests were used to compare the baseline phase to each other phase, within each group.
2.6.6. Chow and sucrose intake at stability during each phase
Absolute chow and sucrose intake were analyzed the same as total food intake (see section 2.6.5.). In addition, stable chow and sucrose intake within the Nicotine C+S group, during the extinction phase, was expressed as a percentage of intake during the treatment phase. These data were not normally distributed; therefore a Wilcoxon test was used to compare the percent change in chow and sucrose intake within the Nicotine C+S group during stable extinction.
2.6.7. Dietary preference during each phase
Stable sucrose intake during baseline, treatment and extinction was calculated as a proportion of total food intake during each phase. A two-way ANOVA was used to analyze differences in the relative proportion of sucrose pellets during stability for each of the three phases between the Nicotine C+S and Saline C+S groups. Holm-Sidak post-hoc tests were used to compare the sucrose proportion of total food intake between the two groups for each phase and Dunnett’s post-hoc tests were used to compare phases within the groups.
2.6.8. Nicotine C-Only
Because total food intake, weight gain, and nicotine self-administration were similar between the Nicotine C+S and Nicotine C-Only groups (see supplementary materials), only the Nicotine C+S and Saline C+S groups are shown in most figures for clarity.
Results
3.1 Nicotine self-administration
Figure 1 shows the mean number of infusions earned throughout the treatment and extinction phases for all three groups. During the first 10 days of treatment, there was a main effect of group (F(2, 22) = 7.6; p < 0.01) and day (F(10, 220) = 7.7; p < 0.01) on mean number of infusions. There was no significant group × day interaction. Post-hoc tests revealed that the mean number of infusions obtained between the two nicotine groups did not differ. Both the Nicotine C+S and Nicotine C-Only groups earned more infusions compared to the Saline C+S group. There was no difference in the mean ± SEM number of days it took to acquire NSA (defined as the number of days until stable) between the Nicotine C+S (22.6 ± 2.5) and Nicotine C-Only (19.5 ± 3.2) groups.
Fig. 1.
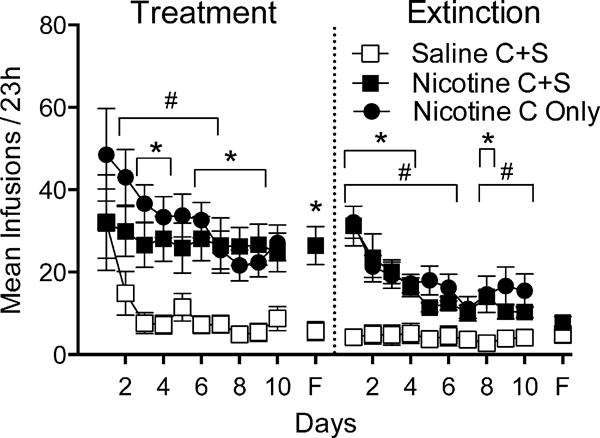
Mean (±SEM) number of infusions earned during the treatment and extinction phases for rats in the Saline C+S, Nicotine C+S, and Nicotine C-Only groups. The left half depicts the mean +/− SEM number of infusions earned during the first 10 days of the treatment phase and the last day of the treatment phase (F). The right half depicts the mean ± SEM number of infusions earned during the first 10 days of the extinction phase and the last day of the extinction phase (F). *p< 0.05 difference between Nicotine C+S and Saline C+S on the indicated day(s). #p < 0.05 difference between Nicotine C Only and Saline C+S on the indicated day(s).
During the first 10 days of extinction (Figure 1), there was a main effect of group (F(2, 22) = 13.8; p < 0.01) and day (F(10, 220) = 11.6; p < 0.01) on the mean number of infusions, and a significant group × day interaction (F(20, 220) = 3.1; P < 0.01). Post-hoc tests revealed that the mean number of infusions obtained between the two nicotine groups did not differ at any time point. The Nicotine C+S and Nicotine C-Only groups obtained more infusions compared to the Saline C+S group. There were no differences between the nicotine groups and the Saline C+S group on the final day of extinction, verifying extinction of lever pressing in the nicotine groups.
3.2. Body weight
Figure 2 shows the percent change in body weight over the first 10 days of treatment (Figure 2a) and extinction (Figure 2b). The rate of weight gain over the final four days of the baseline period (data not shown) did not differ between the three groups, although the Nicotine C-Only group gained more weight on the final day of the baseline phase compared to the Nicotine C-S and Saline C-S groups. Mean weight gain (± SEM) was 3.2% ± 1.1, 3.1% ± 1.1, and 5.4% ± 1.7, for the Saline C+S, Nicotine C+S, and Nicotine C-Only groups, respectively. During the first 10 days of treatment (Figure 2a), there was a significant main effect of group (F(2, 22) = 19.1; p < 0.01) and day (F(10, 220) = 11.5; p < 0.01) on weight gain, and a significant group × day interaction (F(20, 220) = 5.8; p < 0.01). Post-hoc tests revealed that there was no significant difference in weight gain between the two nicotine groups at any time point. There was a significant decrease in weight gain on all but the first day of the treatment phase in the Nicotine C+S group compared to the Saline C+S group, and decreased weight gain on all but the first two days of treatment in the Nicotine C-Only group compared with the Saline C+S, indicating a decrease in daily weight gain during NSA. In addition, linear regression analyses showed a significant difference in slope between the Saline C+S group and the Nicotine C+S (F(1, 183) = 34.1; p < 0.01) and Nicotine C-Only (F(1, 172) = 31.1; p < 0.01) groups during the first 10 days of the treatment phase, indicating that NSA decreased the overall rate of weight gain.
Fig. 2.
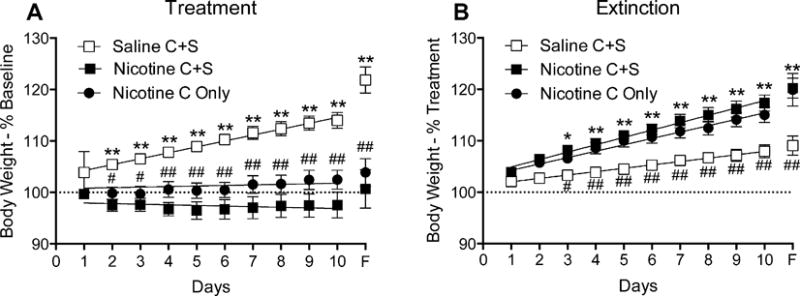
Mean (± SEM) percent change in body weight over the first 10 days of the treatment phase (panel A) and the first 10 days of the extinction phase (panel B) for rats in the Saline C+S, Nicotine C+S, and Nicotine C-Only groups. Values were calculated relative to the mean body weight during the final five days of the previous phase: treatment weight gain was calculated relative to body weight in the final five days of the baseline phase and extinction weight gain was calculated relative to body weight during the final five days of the treatment phase. *p < 0.05, **p < 0.01 difference in % change weight gain between Nicotine C+S and Saline C+S. #p < 0.05, ##p < 0.01 difference in % change weight gain between Nicotine C Only and Saline C+S.
During the first 10 days of extinction (Figure 2b), there was a significant main effect of group (F(2, 22) = 10.7; p < 0.01) and day (F(10, 220) = 127.5; p < 0.01) on daily weight gain, and a significant group × day interaction (F(20, 220) = 6.2; p < 0.01). Post-hoc tests revealed that there was no significant difference in weight gain between the two nicotine groups during this period. There was a significant increase in daily weight gain between the Nicotine C+S group and the Saline C+S group after day two, and increased daily weight gain in the Nicotine C-Only group compared with the Saline C+S group after day three, indicating that withdrawal from nicotine increased daily weight gain. In addition, linear regression analyses between the groups revealed a significant difference in slope between the Saline C+S group and the Nicotine C+S (F(1, 183) = 28.5; p < 0.01) and Nicotine C-Only (F(1, 172) = 18.6; p < 0.01) groups during the extinction phase, indicating that NSA withdrawal increased the overall rate of weight gain.
3.3. Food intake
3.3.1. Daily time course of total food intake
Figure 3a shows the mean total food intake for the Nicotine C+S and Saline C+S groups at baseline and during each of the first 10 days and the final day of the treatment and extinction phases. There was a significant main effect of group (F(2,22) = 28.78; p < 0.01) and day (F(12,264) = 9.27; p < 0.01) on total food intake during the treatment phase, and a significant group by day interaction (F(24,262) = 3.22; p < 0.01). There was no significant difference in total food intake in the Saline C+S group on any of the treatment days relative to baseline. In contrast, total food intake in the Nicotine C+S group was decreased throughout the treatment phase, relative to baseline. There were significant group differences in total food intake during the treatment phase, indicating that nicotine self-administration reduced total food intake.
Fig. 3.
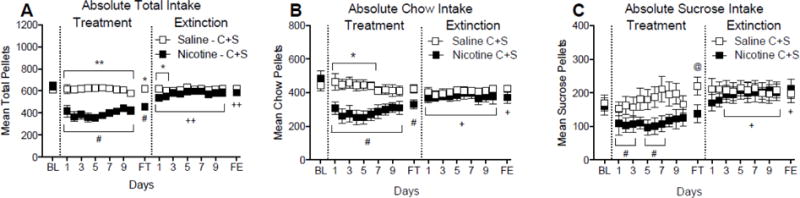
Mean (± SEM) of total food intake over the treatment and extinction phases for rats in the Saline C+S and Nicotine C+S groups. Shown is the mean ± SEM total food intake (panel A), chow intake (panel B), and sucrose intake (panel C) during the last day of the baseline phase (BL) and the first 10 days and the last day (F) of the treatment and extinction phases. *p < 0.05, **p < 0.01 difference between Nicotine C+S and Saline C+S on the indicated day(s). # p < 0.05, ##p < 0.01 difference from baseline in the Nicotine C+S group. +p < 0.05; ++p < 0.01 difference from final treatment days. @p < 0.05 difference from baseline in the Saline C+S group.
There was a significant main effect of day (F(12,264) = 10.86; p < 0.01), but not group, on total food intake during the extinction phase. There was also a significant group by day interaction (F(24,264) = 2.26; p <0.01) during the extinction phase. Compared to total food intake at the end of the treatment phase, there were no significant differences in total food intake in the Saline C+S group. In contrast, the Nicotine C+S group consumed more total food throughout the extinction phase, relative to the treatment phase. There were significant group differences on only the first two days of the extinction phase, indicating that total food intake returned back to control levels.
3.3.2. Daily time course of absolute chow and sucrose intake
Figure 3b shows absolute chow intake at baseline and during each of the first 10 days and final day of the treatment and extinction phases. There was a significant main effect of group (F(2,22) = 11.03; p < 0.01) and day (F(12,264) = 7.42; p < 0.01) on chow intake during the treatment phase, and a significant group by day interaction (F(24,262) = 2.81; p < 0.01). Absolute chow intake in the Nicotine C+S group was decreased on all days of the treatment phase, relative to baseline. Significant group differences in chow intake were evident in the first week of the treatment phase. During the extinction phase, there was a significant main effect of group (F(2,22) = 18.10; p < 0.01) and day (F(12,264) = 7.60; p < 0.01) on chow intake, and a significant group by day interaction (F(24,262) = 2.01; p < 0.01). Compared to the treatment phase, chow intake was increased on all days of the extinction. However, there was no significant difference in chow intake between groups, indicating that chow intake returned to saline controls levels.
Figure 3c shows the mean absolute sucrose intake at the same points as Figure 3a. There was a significant main effect of day (F(11,165) = 2.89; p < 0.01), but not group, on sucrose intake during the treatment phase, and a significant group by day interaction (F(11,165) = 2.01; p < 0.01). There was a significant difference in the Saline C+S group on the final day of the treatment phase, relative to the final five days of baseline, indicating a natural rise in sucrose intake over time in the control group. Compared to baseline, absolute sucrose intake in the Nicotine C+S group was transiently decreased during the first week, but not during the final days of the treatment phase, suggesting tolerance or resistance to nicotine’s suppressant effects on sucrose intake. There were no significant differences in daily sucrose intake between the Nicotine C+S and Saline C+S groups during the treatment phase. There was a significant main effect of day (F(11,165) = 2.14; p < 0.05), but not group, on sucrose intake during the extinction phase, and a significant group by day interaction (F(11,165) = 2.94; p < 0.01). Sucrose intake did not change during extinction in the Saline C+S Group, relative to intake during treatment, on any day. Compared to the treatment phase, absolute sucrose intake was increased during all but the first day of the extinction phase in the Nicotine C+S group. There were no significant differences in daily sucrose intake between groups during treatment.
3.3.2. Total food intake at stability during each phase
Figure 4 shows the mean total food intake at the end of each phase in the Saline C+S and Nicotine C+S groups. There was a significant main effect of phase (F(2, 44) = 23.0; p < 0.01), but not group, and a significant group × phase interaction (F(4, 44) = 5.8; p < 0.01). Post-hoc tests revealed there were no differences in total food intake across phases in the Saline C+S group. Within group comparisons indicated a significant decrease in total food intake during treatment compared to baseline and extinction in the Nicotine C+S group, and a persistent decrease in food intake during extinction relative to baseline, indicating that total food intake did not return to baseline levels. There was a significant difference in total food intake during the treatment phase between the Nicotine C+S and Saline C+S groups. There was no difference between groups in total food intake during the baseline and extinction phases.
Fig. 4.
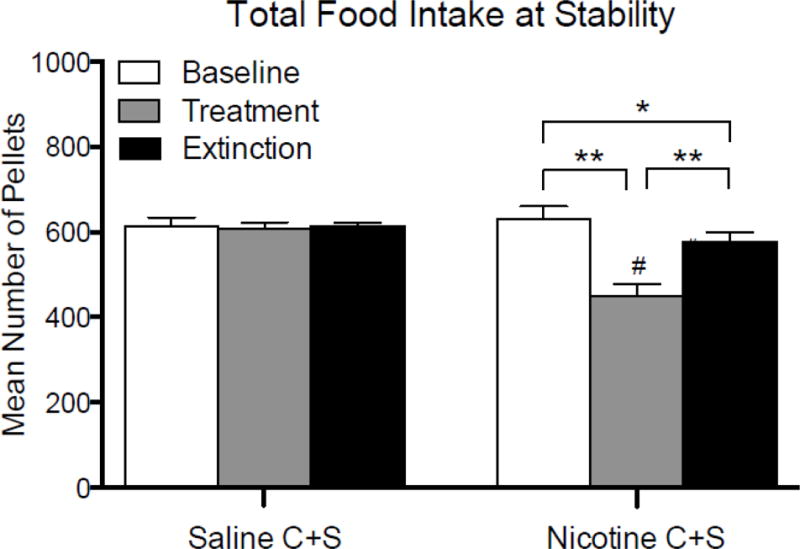
Mean (± SEM) total pellets earned during the final five days of the baseline, treatment, and extinction phases for the Saline C+S and the Nicotine C+S groups. *p < 0.05, **p < 0.01 difference between phases within the Nicotine C+S group. #p < 0.05 difference between the Nicotine C+S and saline C+S groups for the indicate phase.
3.3.3. Absolute chow and sucrose intake at stability during each phase
Figure 5a shows absolute chow and sucrose intake for the Nicotine C+S and Saline C+S groups during the final five days of each phase. There was a significant main effect of phase (F(2, 44) = 29.3; p < 0.01) and group (F(2, 22) = 11.1; p < 0.01), on absolute chow intake. There was also a significant phase × group interaction (F(4, 4) = 3.0; p < 0.05). Post-hoc tests revealed there was no difference in chow intake in the Saline C+S group for any of the phases. There was a significant decrease in chow intake during the treatment and extinction phases compared to the baseline phase in the Nicotine C+S group, and a significant increase in chow intake during extinction compared to treatment. There was no significant difference in chow intake between the Nicotine C+S and Saline C+S groups for any of the phases.
Fig. 5.
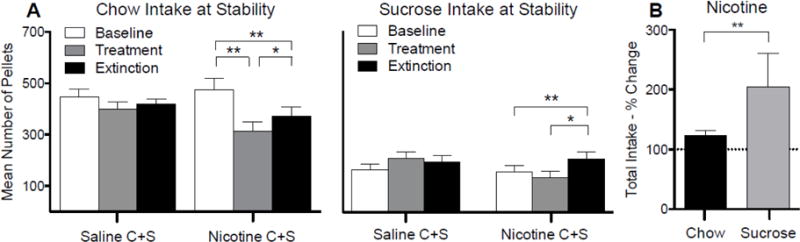
Mean (± SEM) chow and sucrose pellets earned during the final five days of the baseline, treatment, and extinction phases for the Nicotine C+S and Saline C+S (panel A) groups. Also shown is the mean ± SEM percent change in chow and sucrose intake in the Nicotine C+S group during the final five days of the extinction phase. Percent change was calculated relative to the mean intake during the final five days of the treatment phase. *p < 0.05, **p < 0.01 difference in intake between the indicated phases.
There was a significant main effect of phase (F(2, 30) = 6.0; p < 0.01), but not group, on absolute sucrose intake, and a significant phase × group interaction (F(2, 30) = 6.4; p < 0.01). Post-hoc tests revealed there was no change in sucrose intake across phases in the Saline C+S group. There was no significant difference in sucrose intake in the Nicotine C+S group between the baseline and treatment phases. There was a significant increase in sucrose intake in the Nicotine C+S group during extinction relative to baseline and during treatment. There was no difference in sucrose intake between groups during any phase.
Figure 5b shows the percent change in sucrose and chow intake during extinction relative to the treatment phase for the Nicotine C+S group. The percent increase in sucrose intake (Median: 150.9%; Range: 94.7 – 782.3%) during extinction was significantly greater than the percent increase in chow intake (Median: 124.9%; Range: 85.9 – 227.1%) during this time (W = 43.00; p < 0.01), indicating a preferential increase in sucrose intake following withdrawal from nicotine.
3.3.4. Dietary preference during each phase
Figure 6 shows stable sucrose intake during each phase, expressed as a proportion of total food intake, in each group. There was a significant main effect of phase (2, 30) = 6.5; p < 0.01), but not of group, on the proportion of sucrose pellets during each phase. There was no significant phase × group interaction. There was no change in the proportion of sucrose pellets consumed between any of the phases for the Saline C+S group. There was no significant change in the proportion of sucrose pellets in the Nicotine C+S group during the treatment phase. However, there was a significant increase in the proportion of sucrose pellets consumed during the extinction phase, compared to baseline in the Nicotine C+S group. There was no difference in the proportion of sucrose pellets between the groups for any phase.
Fig. 6.
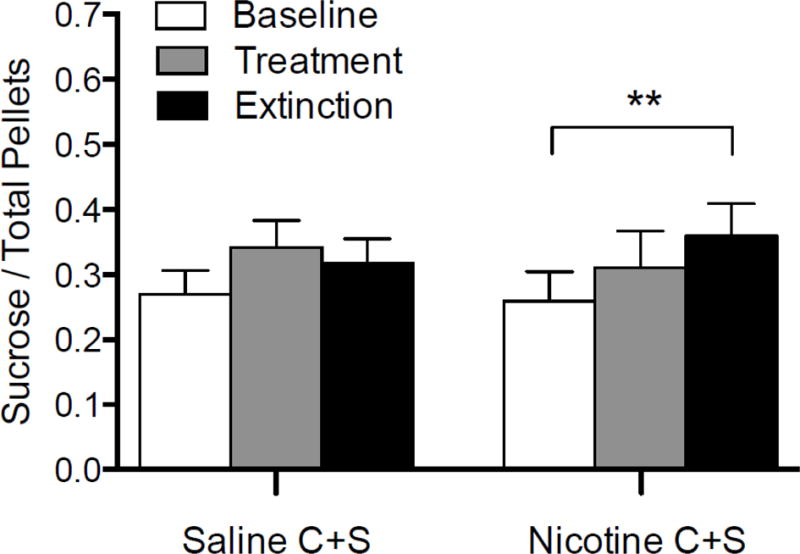
Mean (± SEM) sucrose intake during the final five days of baseline, treatment, and extinction for the Saline C+S and Nicotine C+S groups, expressed as a proportion of total intake. **p < 0.01 difference between indicated phases for the Nicotine C+S group.
Discussion
To our knowledge, this is the first study to examine the differential effects of self-administered nicotine on concurrent intake of different food types. In summary, the present study found that NSA reduced food intake, whereas extinction of NSA (i.e., nicotine withdrawal) increased food intake. Compared to chow intake, sucrose intake was more resistant to the effects of NSA, but more sensitive to the effects of withdrawal, such that the percent increase in sucrose intake was greater than the percent increase in chow intake during withdrawal. In addition, a larger proportion of calories were consumed from sucrose during nicotine withdrawal than were consumed prior to nicotine administration. Consistent with these changes in food intake, weight gain was slower during NSA and faster during withdrawal.
4.1. Nicotine self-administration
4.1.1. Effects of food availability on NSA
The Nicotine C+S and Nicotine C-Only groups earned a similar number of infusions during the treatment phase, indicating that concurrent free access to an alternative reinforcer does not reduce NSA. This is consistent with other studies demonstrating that concurrent access to sucrose and nicotine in limited access sessions does not decrease rates of NSA [50,51], unless sucrose availability is contingent upon abstaining from nicotine [50]. Moreover, rats in the present study showed robust NSA despite having ad libitum access to food, which is consistent with other studies showing that food deprivation is not required for nicotine to serve as a reinforce [52,53].
4.1.2. Effects of NSA on total food intake and body weight
O’Dell et al. (2007) was the first study to characterize the effects of self-administered nicotine on changes in food intake. Similar to what was described that study, NSA in the present study resulted in a decrease in bland food intake. However, the time course of daily changes in weight gain were not compared against a saline control group and withdrawal effects were not examined in that study. The present study extends the findings from O’Dell et al. (2007) by showing that both daily body-weight gain and food intake decrease during NSA and increase following withdrawal from nicotine. In addition, the present study shows that the effects of NSA and withdrawal are moderated by the availability of multiple food types (see sections 4.1.3. and 4.2.2.).
The decrease in body weight produced by NSA in the present study is similar to that produced by noncontingent nicotine administration reported in other studies [17,35,39,45]. However, the effects of NSA on total food intake differed somewhat from previous studies using noncontingent systemic nicotine administration. Although the reduction in total food intake in the present study was similar to what’s been seen during the first few days of noncontingent intermittent i.p. or continuous s.c. nicotine administration [17,39], the lack of tolerance in both nicotine groups in the present study contrasts with these previous studies. The different routes of administration may account for these discrepancies. The effective level of nicotine exposure in the present study and a previous noncontingent i.v. nicotine administration study (~1.5 mg/kg/day; [45]) was significantly lower than the daily nicotine exposure required to induce changes in food intake during noncontingent continuous infusion or multiple, daily i.p. injections (12.00 or 4.00 mg/kg, respectively; [35,39]). Thus, because the degree of tolerance is usually greater with more continuous exposure to higher doses [54], the lower tolerance in the present study would be expected. However, serum and brain nicotine concentrations were not measured in the present study, and such concentrations following i.v. infusions could be similar to that achieved with higher daily doses from continuous or intermittent s.c. administration [41,55].
Several other factors may contribute to discrepancies between the present and previous studies. First, because total food intake in prior investigations was measured as changes in chow intake only (i.e., it was the only food available), it is possible that tolerance to nicotine’s effects depends upon the number of available foods. However, the lack of tolerance effects in both nicotine self-administration groups in the present study (see also: supplementary materials) does not support such interpretation. Second, the contingency of drug administration may be an important factor. Noncontingent nicotine administration produces an enhanced stress response, as seen by persistent elevations of corticosterone [49]. Thus, because it can alter levels of feeding neuropeptides [56] and suppress food intake [57], stress may contribute to the effects of noncontingent nicotine administration. Furthermore, because smoking tends to reduce stress in humans [58,59], nicotine dosing regimens that can induce stress are less desirable for studying food intake. Third, compared to prior studies, the effort to consume food was somewhat greater in the present study because of an imposed operant response requirement, which might lead to a slower rate of food consumption compared to that when food is available en mass. Because effort and reinforcement rate or delay can modulate tolerance to drug effects on food maintained behavior [60–62], this may account for differences in the degree of tolerance across studies. Finally, differences in rat strain and age between studies may also be important factors.
4.1.3. Effects of NSA on chow and sucrose intake
The present study extends O’Dell et al. (2007) and our previous study [63], by describing the effects of self-administered nicotine on concurrent intake of different types of food. Because human smokers have concurrent access to different freely available foods, the present study improves the ecological validity of animal models to examine factors that mediate the relationship between nicotine and food intake. Total food intake and weight gain during the baseline and treatment phases did not differ between the two nicotine groups, indicating that total caloric intake was not influenced by sucrose availability. The proportion of food intake from sucrose at baseline in the Nicotine C+S and Saline C+S groups was 26% and 27%, respectively. This falls within the percentage of calories from sugar consumed by humans, which is around 15% in adults [64,65] and as high as 40% in children and adolescents [66]. These findings support the external validity of the present model.
The duration of nicotine’s effects on food intake was dependent upon the type of food available, in that tolerance developed to a greater degree for sucrose intake compared to chow intake. The resistance of sweet food intake to nicotine’s anorectic effects suggests that decreased weight gain in smokers may be attributable, in part, to a selective decrease in non-sweet food intake. In addition, the trend for the proportion of sucrose intake to increase during NSA and further increase during withdrawal, suggests that increased sugar preference is a potential mechanism for the increased risk of insulin resistance seen in human smokers [67–69]. This has important implications for understanding the development and treatment of health problems associated with post-cessation weight gain. Specifically, that interventions targeting carbohydrate intake may be important.
Few studies have examined the effects of noncontingent nicotine on the intake of multiples food types using an animal model, and our findings bare some similarities and differences to them. In Grunberg et al. (1985), male rats were given ad libitum access to nonsweet chow and sweetened chow. Nicotine was administered via minipumps and food intake and body weight was averaged over a stable period of intake and within the first six days after the pump was removed. Whereas weight gain decreased in animals with concurrent access to nonsweet and sweet foods in the present study, weight gain was largely unchanged in Grunberg et al. (1985). The relative resistance of sweet food intake to nicotine was consistent with the transient decrease in sweet food intake in the present study. However, in Grunberg et al. (1985), bland chow intake during nicotine administration was not decreased at all. Similar to the differences in total intake between this and other studies, the differences in sucrose intake and weight gain may be due to differences in the nicotine dosing regimen (high dose continuous infusion v. low dose self-administration) and the route of administration (subcutaneous v. intravenous), which may have led to differences in serum nicotine levels and stress hormones. For example, chronic stress has been shown to produce distinct changes in carbohydrate metabolism and weight gain [70,71]. Another difference between the studies was the composition of the diet. The sweet food in Grunberg et al. (1985) was identical in composition to the nonsweet food, except for the addition of saccharin to increase sweetness. In contrast, the current study used sucrose pellets, and it is possible that the relative palatability of the alternative foods differed between studies. Finally, access to food also differed somewhat between the studies. In the current study, an operant response requirement was imposed for animals to obtain food, whereas unlimited food was presented all at once in the Grunberg et al. (1985) experiment. These differences in protocols could account for the differences in food intake between the two studies.
4.2. Nicotine Withdrawal
4.2.1. Effects of extinction on total food intake and body weight
Both nicotine groups gained weight at a faster rate during nicotine withdrawal, compared to the saline group. This change was also reflected in the absolute mean weight gain from the last day of the treatment phase to the last day of extinction for the Nicotine C+S and Nicotine C-Only groups that gained an average of 69g and 78g, respectively, compared to ~41g in the Saline C+S group during this period. While the absolute body weights (mean ± SEM) for the Nicotine C+S (406g ± 6.5g) and Nicotine C-Only (412g ± 6.0g) groups at the end of extinction were not back up to or above saline control levels (518g ± 4.0g), as has been reported within the human literature [16], this is not unexpected. Rodents, as opposed to humans, continue to grow well into adulthood [72]. Therefore, weight gain was analyzed as a rate of weight change in the present study.
The observed increase in total food intake during extinction in the present study provides further support for the notion that increased weight gain following smoking cessation is largely attributable to increased food intake. Withdrawal mediated increases in food intake in the present study were generally similar to previous investigations in which nicotine was administered noncontingently [17,37,45]. Withdrawal from both self-administration and noncontingent administration produced increases in total food intake back to control levels [17,19,37,39,45,63]. However, weight gain was not increased relative to controls during nicotine withdrawal following i.p. injections, despite food intake returning back to baseline levels [39]. Withdrawal-mediated increases in total food intake and weight gain more closely resembling the effects described in the present study, have been seen in investigations where nicotine was administered continuously through minipumps [37]. As previously discussed, discrepancies in route or contingency of administration could influence changes in nicotine metabolism and stress that may lead to differential effects once treatment is stopped. Likewise, nicotine dose may play an important role in post-cessation weight gain, as increases in weight gain during withdrawal in previous studies were seen only when very high doses of nicotine were administered continuously (9 mg/kg/day; [37]).
4.2.2. Effects of extinction on chow and sucrose intake
In the present study, there was an increase in both chow and sucrose intake during withdrawal from NSA. In contrast, Grunberg et al. (1985) failed to show an increase in bland chow intake alongside the increase in sweet food intake during the first six days of withdrawal. It is unknown whether these changes would have persisted, as withdrawal effects were not measured beyond this period. Nevertheless, both studies suggest that withdrawal from nicotine produces a greater increase in sweet food consumption, suggesting the selective effect of nicotine withdrawal on sucrose intake generalizes across different models of nicotine exposure.
Although both chow and sucrose intake was increased during withdrawal, the proportion of sucrose consumed was greater relative to chow. This increase in the proportion of sucrose in the diet may have important clinical implications. Meta-analyses have shown an association between increased sugar intake and increased body weight (and vice versa), and an increased odds ratio (1.55) of becoming obese in individuals who consume high amounts of sugar-sweetened beverages [73]. The proportion of sucrose intake consumed in the present study increased from baseline by 10% (from 26% to 36%), a particularly large increase for such a short period of time and twice the increase compared to the Saline C+S group (5%; from 27% to 32%). It is possible that a similar increase in ex-smokers could limit or delay the benefits of quitting smoking by increasing their risk for obesity-related diseases [74,75]. For example, it may take ex-smokers >10 years to decrease their risk for diabetes [76,77] coronary heart disease [78], hypertension [79,80], and impaired lung function [81], because the beneficial effect of quitting smoking decreases proportionally for every kilogram of weight gained [82,83]. The animal model in the present study provides a means for investigating potential mechanisms underlying the selective changes in carbohydrate intake associated with nicotine withdrawal and treatments targeting this aspect of food intake to reduce post-cessation weight gain.
4.3. Potential mechanisms
4.3.1. Reinforcement enhancement
The findings from the present study are seemingly inconsistent with previous research demonstrating that nicotine enhances the reinforcing efficacy of sucrose and that withdrawal from nicotine leads to reward devaluation. Numerous studies have shown that acute noncontingent nicotine administration increases responding for sucrose under fixed-ratio [84] and progressive-ratio schedules during limited access to nicotine [85–87]. In contrast, NSA in the present study only produced a transient decrease in sucrose intake. One reason for this discrepancy may be that unlimited food intake is relatively insensitive to increases in reinforcer efficacy because it allows satiation to occur, which decreases the reinforcing efficacy of food. Reinforcement enhancement per se is best studied when access is limited enough to avoid confounding effects of satiation. Therefore, the present findings may better reflect the effects of nicotine on homeostatic mechanisms regulating food intake (e.g., satiety and related feeding neuropeptides).
4.3.2. Central nervous system mechanisms
In human smokers, the direct effects of nicotine per se on food reinforcement are confounded by the effects of cigarette smoke on taste thresholds via interaction with taste receptors in the mouth. For example, electrophysiological studies have found that a majority of smokers have elevated taste thresholds and fewer and flatter papillae compared to nonsmokers [88]. Furthermore, dose pack years are positively correlated with taste thresholds [34] and negatively correlated with the ability to correctly identify odors in current and past smokers [89]. However, switching to reduced nicotine content cigarettes lowers taste thresholds and increases self-reported eating, suggesting that the central effects of nicotine can influence food intake [31,90,91]. The present study provides further support for this notion. Because nicotine was administered intravenously, changes in sucrose intake were due to the central effects of nicotine, independent of peripheral effects on gustatory receptors.
The observed changes in total food intake in the present study may be mediated through nicotine’s actions on feeding-related central or peripheral neural mechanisms. For example, nicotine may act on nicotinic acetylcholine receptors (nAChRs) located on trigeminal afferents in the oral cavity, the nucleus of the solitary tract (NTS), or on Neuropeptide-Y (NPY) and proopiomelanacortin (POMC) neurons within the hypothalamus. (For a comprehensive review on the role of nicotine and nAChRs in energy homeostasis, see [92] and [93].) Studies evaluating the effects of self-administered nicotine could help clarify nicotine’s specific effects on these systems, while minimizing its effects on stress-related systems. Following nicotine cessation, a potential target for the specific changes in carbohydrate intake seen in the present study are withdrawal-mediated decreases in serotonin. Because nAChR’s are located on serotonergic neurons and their activation increases serotonin release [94] and inhibits the production of NPY [95], removal of this inhibition following smoking cessation may lead to a compensatory increase in carbohydrate intake. Finally, orexin signaling in arcuate nucleus may be of interest, given the increase in orexin A mRNA that has been observed after NSA [96]. Future studies will be helpful for clarifying the relative contribution of these systems.
4.3.3. Relief of withdrawal
Nicotine may also alter carbohydrate intake through behavioral mechanisms. For example carbohydrates may serve as a substitute for nicotine during withdrawal. The loss of one reinforcer (e.g., nicotine) could increase responding for another available reinforcer (e.g., sucrose), depending on the extent to which the two reinforcers were economic substitutes for one another [97]. The increase in sucrose preference during withdrawal suggests that sweet foods may be better able to substitute for nicotine. The increase in sucrose preference in the present study is also similar to human studies showing that carbohydrate intake is positively correlated with withdrawal symptoms during smoking abstinence [30,34,47], suggesting that individuals may attempt to mitigate withdrawal symptoms by increasing their carbohydrate intake. Consistent with this notion, human clinical data have shown that chewing glucose tablets can increase abstinence from smoking when combined with traditional pharmacotherapies [98] and reduce the severity of withdrawal symptoms [99]. Animal models of nicotine self-administration can be used to determine the extent to which nicotine and sucrose are economic substitutes for one another and the underlying neural mechanisms of this relationship.
4.4. Limitations
There are several limitations of the present study. First, because animals in the Nicotine C+S group were underweight, it is unknown to what extent the increase in sucrose preference was driven by withdrawal from nicotine per se or the history of reduced food intake and body weight. Although there was no pair-fed or weight matched group to control for this possibility, this seems unlikely. Food restriction has been shown to increase preference for palatable non-sweet nutrients (e.g., starch, fat) over iso-caloric sweet nutrients (e.g., [100,101]). Therefore, since chow was a relatively non-sweet nutrient and the only source of fat in the present study, preference for sucrose would be expected to be lower in food-restricted pair-fed controls. However, it will be important to examine whether other means of reducing food intake and body weight (e.g., noncontingent of self-administered exposure to other stimulants) might modulate sucrose preference to determine whether the present findings are specific to nicotine.
Second, these results cannot be generalized to nicotine’s effects on more calorically dense foods. Providing access to sucrose pellets that were calorically similar to chow pellets in the present study allowed for the examination of nicotine’s effects on intake of sweet tasting food per se, by controlling for the potential confound of increased caloric content. However, it is likely that the sweet foods consumed by humans are higher in calories, and this is not accounted for in the present model. Second, although concurrent access to two different types of food was arranged, we cannot rule out that changes in food intake, body weight, and NSA would be differentially affected had we provided access to highly palatable, high fat foods in addition to or instead of sucrose. Further increasing the variety of available food would more accurately simulate the vast array of food choices available to human smokers.
Third, because only one dose of nicotine was self-administered in the current study, the dose response relationship between self-administered nicotine and sucrose intake is unknown. In light of potential FDA-mandated reductions in the nicotine content of tobacco products [102–104], characterizing the effects of low doses of nicotine on food intake and weight gain will be particularly important. Given the well-documented relationship between quitting smoking and weight gain [5,13,16], it is possible that reductions in the content of nicotine in cigarettes could produce population-wide increases in obesity and obesity-related disease.
Fourth, the rates of self-administration in the present study are comparable to the upper limit of what has been reported in individual smokers (up to 1.38 mg/kg; [105]). Because the human literature suggests that nicotine’s effects on body weight are dose dependent [12,27,106], the results from the present study may most relevant to changes in body weight and food intake in heavier smokers.
Lastly, sex differences in NSA, food intake, and weight gain were not examined. It has been well documented in smokers that post-cessation weight gain is the largest in females and that fear of weight gain is a significant barrier to quitting in females. Studying females in this area will be critical to a comprehensive assessment of the individual variability in the effects of nicotine and withdrawal on food intake and weight gain and their underlying mechanisms.
Conclusions
Despite its limitations, the present study advances animal models of smoking’s effects on food intake and body weight. Primary advantages of the model include a) allowing nicotine to be self-administered concurrently with multiple types of food, as it is in smokers, b) achieving doses and patterns of nicotine administration and resulting serum and brain nicotine concentrations that are more comparable to smoking, and c) avoiding confounding stress effects of noncontingent nicotine administration. The decreased food intake and weight gain during nicotine administration and increased intake and weight gain during withdrawal observed in the present model more consistently replicates findings from the human literature, compared to noncontingent delivery models. The observation of differential effects of NSA withdrawal on sucrose intake supports the notion that weight gain prevention and treatment options should focus on the mechanisms underlying increases in carbohydrate intake.
Supplementary Material
Highlights.
Nicotine’s effects on body weight, chow, and sucrose intake were modeled in rats
Nicotine self-administration decreased body weight and chow intake
Tolerance to nicotine’s effects on food intake developed for sucrose, but not chow
Withdrawal from nicotine resulted in increased weight gain and food intake
Withdrawal-mediated increases in sucrose were greater than increases in chow
Acknowledgments
The authors thank Dr. Andrew Harris for his comments on earlier versions of the manuscript. Support for this study was provided by NIH/NIDA grant R01-026444 (MGL) and a Career Development Award (MGL) and Postdoctoral Fellowship (PEB) from the Minneapolis Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses — United States, 2000–2004, MMWR. Morbidity and Mortality Weekly Report. 2008;57:1226. [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Cawley J, Meyerhoefer C. The medical care costs of obesity: An instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Revs. 2010;12:50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filozof C, Fernández Pinilla MC, Fernández-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 6.Hughes J, Kee F, O’Flaherty M, Critchley J, Cupples M, Capewell S, et al. Modeling coronary heart disease mortality in Northern Ireland between 1987 and 2007: broader lessons for prevention. Eur J Prev Cardiol. 2013;20:310–321. doi: 10.1177/2047487312441725. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Eriksson H, Larsson B, Svärdsudd K, Tibblin G, Welin L, et al. Secular changes in cardiovascular risk factors over 30 years in Swedish men aged 50: the study of men born in 1913, 1923, 1933 and 1943. J Intern Med. 2000;247:111–118. doi: 10.1046/j.1365-2796.2000.00589.x. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333:1165–1170. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 9.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. Bmj. 2012;345:e4439–e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshminarayanan M, Casey DE, Allison DB. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277–288. doi: 10.1016/s0165-1781(01)00234-7. [DOI] [PubMed] [Google Scholar]
- 11.Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–217. [PubMed] [Google Scholar]
- 12.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 13.Audrain-McGovern J, Benowitz NL. Cigarette Smoking, Nicotine, and Body Weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamford BA, Matter S, Fell RD, Papanek P. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr. 1986;43:486–494. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg NE, Bowen DJ, Winders SE. Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl) 1986;90:101–105. doi: 10.1007/BF00172879. [DOI] [PubMed] [Google Scholar]
- 16.Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 17.Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav. 2001;74:169–176. doi: 10.1016/s0031-9384(01)00540-6. [DOI] [PubMed] [Google Scholar]
- 18.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellinger LL, Wellman PJ, Harris RBS, Kelso EW, Kramer PR. The effects of chronic nicotine on meal patterns, food intake, metabolism and body weight of male rats. Pharmacol Biochem Behav. 2010;95:92–99. doi: 10.1016/j.pbb.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Bradley DP, Johnson LA, Zhang Z, Subar AF, Troiano RP, Schatzkin A, Schoeller DA. Effect of smoking status on total energy expenditure. Nutr Metab (Lond) 2010;7:81. doi: 10.1186/1743-7075-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellerstein MK, Benowitz NL, Neese RA, Schwartz JM, Hoh R, Jacob P, Hsieh J, Faix D. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest. 1994;93:265–272. doi: 10.1172/JCI116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med. 1986;9:79–82. doi: 10.1056/NEJM198601093140204. [DOI] [PubMed] [Google Scholar]
- 23.Hur YN, Hong GH, Choi SH, Shin KH, Chun BG. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Mol Cells. 2010;30:219–226. doi: 10.1007/s10059-010-0110-3. [DOI] [PubMed] [Google Scholar]
- 24.Jensen EX, Fusch C, Jaeger P, Peheim E, Horber FF. Impact of chronic cigarette smoking on body composition and fuel metabolism. J Clin Endocrinol Metab. 1995;80:2181–2185. doi: 10.1210/jcem.80.7.7608276. [DOI] [PubMed] [Google Scholar]
- 25.Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr. 1989;50:545–550. doi: 10.1093/ajcn/50.3.545. [DOI] [PubMed] [Google Scholar]
- 26.Hall SM, McGee R, Tunstall C, Duffy J, BENOWITZ N. Changes in food intake and activity after quitting smoking. J Consult Clin Psychol. 1989;57:81–86. doi: 10.2307/2424984?ref=no-x-route:d347287f687b3cb49d1c562cf878f1ee. [DOI] [PubMed] [Google Scholar]
- 27.Rodin J. Weight change following smoking cessation: the role of food intake and exercise. Addict Behav. 1987;12:303–317. doi: 10.1016/0306-4603(87)90045-1. [DOI] [PubMed] [Google Scholar]
- 28.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(Suppl 4):477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 29.Spring B, Wurtman J, Gleason R, Wurtman R, Kessler K. Weight gain and withdrawal symptoms after smoking cessation: a preventive intervention using d-fenfluramine. Health Psychol. 1991;10:216–223. doi: 10.1037//0278-6133.10.3.216. [DOI] [PubMed] [Google Scholar]
- 30.Spring B. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav. 2003;76:351–360. doi: 10.1016/j.pbb.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Mullings EL, Donaldson LF, Melichar JK, Munafo MR. Effects of acute abstinence and nicotine administration on taste perception in cigarette smokers. J Psychopharmacol. 2010;24:1709–1715. doi: 10.1177/0269881109105395. [DOI] [PubMed] [Google Scholar]
- 32.Pomerleau CS, Garcia AW, Drewnowski A, Pomerleau OF. Sweet taste preference in women smokers: comparison with nonsmokers and effects of menstrual phase and nicotine abstinence. Pharmacol Biochem Behav. 1991;40:995–999. doi: 10.1016/0091-3057(91)90118-l. [DOI] [PubMed] [Google Scholar]
- 33.Redington K. Taste differences between cigarette smokers and nonsmokers. Pharmacol Biochem Behav. 1984;21:203–208. doi: 10.1016/0091-3057(84)90215-6. [DOI] [PubMed] [Google Scholar]
- 34.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grunberg NE, Bowen DJ, Maycock VA, Nespor SM. The importance of sweet taste and caloric content in the effects of nicotine on specific food consumption. Psychopharmacology (Berl) 1985;87:198–203. doi: 10.1007/BF00431807. [DOI] [PubMed] [Google Scholar]
- 36.Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 37.Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine’s effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. doi: 10.1016/S0039-6060(99)70163-7. [DOI] [PubMed] [Google Scholar]
- 38.Bellinger L, Cepeda-Benito A, Bullard RL, Wellman PJ. Effect of i.c.v. infusion of the α-MSH agonist MTII on meal patterns in male rats following nicotine withdrawal. Life Sci. 2003;73:1861–1872. doi: 10.1016/S0024-3205(03)00485-5. [DOI] [PubMed] [Google Scholar]
- 39.Bellinger L, Cepeda-Benito A, Wellman PJ. Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol Biochem Behav. 2003;74:495–504. doi: 10.1016/s0091-3057(02)01033-x. [DOI] [PubMed] [Google Scholar]
- 40.Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 41.LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 42.Russell MAH, Feyerabend C, Cole PV. Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br Med J. 1976;1:1043–1046. doi: 10.2307/20409640?ref=no-x-route:3c706ff159e91432a8c6ac837d7030fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winders SE, Grunberg NE, Benowitz NL, Alvares AP. Effects of stress on circulating nicotine and cotinine levels and in vitro nicotine metabolism in the rat. Psychopharmacology (Berl) 1998;137:383–390. doi: 10.1007/s002130050634. [DOI] [PubMed] [Google Scholar]
- 44.Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- 45.Grebenstein PE, Thompson IE, Rowland NE. The effects of extended intravenous nicotine administration on body weight and meal patterns in male Sprague–Dawley Rats. Psychopharmacology (Berl) 2013;228:359–366. doi: 10.1007/s00213-013-3043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol ExpTher. 2006;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- 47.Ogden J. Effects of smoking cessation, restrained eating, and motivational states on food intake in the laboratory. Health Psychol. 1994;13:114–121. doi: 10.1037//0278-6133.13.2.114. [DOI] [PubMed] [Google Scholar]
- 48.Grunberg NE. The effects of nicotine and cigarette smoking on food consumption and taste preferences. Addict Behav. 1982;7:317–331. doi: 10.1016/0306-4603(82)90001-6. [DOI] [PubMed] [Google Scholar]
- 49.Donny EC, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF. Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol. 2000;402:231–240. doi: 10.1016/s0014-2999(00)00532-x. [DOI] [PubMed] [Google Scholar]
- 50.LeSage MG. Toward a nonhuman model of contingency management: effects of reinforcing abstinence from nicotine self-administration in rats with an alternative nondrug reinforcer. Psychopharmacology (Berl) 2009;203:13–22. doi: 10.1007/s00213-008-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panlilio LV, Hogarth L, Shoaib M. Concurrent access to nicotine and sucrose in rats. Psychopharmacology (Berl) 2014;232:1451–1460. doi: 10.1007/s00213-014-3787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 53.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 54.Katz JL, Valentino RJ. Pharmacological and behavioral factors in opioid dependence in animals. In: Goldberg SR, Stolerman IP, editors. Behavioral analysis of drug dependence. Academic Press; New York: 1986. pp. 287–315. [Google Scholar]
- 55.LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- 56.Akabayashi A, Levin N, Paez X, Alexander JT, Leibowitz SF. Hypothalamic neuropeptide Y and its gene expression: relation to light/dark cycle and circulating corticosterone. Mol Cell Neurosci. 1994;5:210–218. doi: 10.1006/mcne.1994.1025. [DOI] [PubMed] [Google Scholar]
- 57.Marti O, Martí J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 58.Kassel JD, Unrod M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J of Abnorm Psychol. 2000;109 doi: 10.1037//002I-843X. [DOI] [PubMed] [Google Scholar]
- 59.Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addict Behav. 1984;9:265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- 61.Nickel M, Poling A. Fixed-ratio size as a determinant of the development of tolerance to morphine. Behav Pharmacol. 1990;1:463. doi: 10.1097/00008877-199000150-00009. [DOI] [PubMed] [Google Scholar]
- 62.Weaver MT, Sweitzer M, Coddington S, Sheppard J, Verdecchia N, Caggiula AR, Sved AF, Donny EC. Precipitated withdrawal from nicotine reduces reinforcing effects of a visual stimulus for rats. Nicotine Tob Res. 2012;14:824–832. doi: 10.1093/ntr/ntr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grebenstein PE, Harp JL, Rowland NE. The effects of noncontingent and self-administered cytisine on body weight and meal patterns in male Sprague–Dawley rats. Pharmacol Biochem Behav. 2013;110:192–200. doi: 10.1016/j.pbb.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brisbois T, Marsden S, Anderson G, Sievenpiper J. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients. 2014;6:1899–1912. doi: 10.3390/nu6051899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 66.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. 2010;110:1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 68.Frati AC, Iniestra F, Ariza CR. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care. 1996;19:112–118. doi: 10.2337/diacare.19.2.112. [DOI] [PubMed] [Google Scholar]
- 69.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinković P, Pešić V, Lončarević N, Smiljanić K, Kanazir S, Ruždijić S. Behavioral and biochemical effects of various food-restriction regimens in the rats. Physiol Behav. 2007;92:492–499. doi: 10.1016/j.physbeh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 71.Zardooz H, Zahdiasl S, Gharibnaseri M, Hedayati M. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol Behav. 2006;89:373–378. doi: 10.1016/j.physbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Swanson HE, van der Werff Ten Bosch J. Sex differences in growth of rats, and their modification by a single injection of testosterone propionate shortly after birth. J Endocrinol. 1963;26:197–207. doi: 10.1016/j.apergo.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 73.Morenga LT, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Bmj. 2013;346:e7492–e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 74.Laguna J, Alegret M, Roglans N. Simple sugar intake and hepatocellular carcinoma: epidemiological and mechanistic insight. Nutrients. 2014;6:5933–5954. doi: 10.3390/nu6125933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siervo M, Montagnese C, Mathers JC, Soroka KR, Stephan BC, Wells JC. Sugar consumption and global prevalence of obesity and hypertension: an ecological analysis. Public Health Nutr. 2013;17:587–596. doi: 10.1017/S1368980013000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camp DE, Klesges RC, Relyea G. The relationship between body weight concerns and adolescent smoking. Health Psychol. 1993;12:24–32. doi: 10.1037//0278-6133.12.1.24. [DOI] [PubMed] [Google Scholar]
- 77.Luo J, Rossouw J, Tong E, Giovino GA, Lee C, Chen C, Ockene JK, Qi L, Margolis KL. Smoking cessation, weight gain, and risk of type 2 diabetes mellitus among postmenopausal women. Arch Intern Med. 2012;172:438–440. doi: 10.1001/archinternmed.2012.24. [DOI] [PubMed] [Google Scholar]
- 78.Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002;53:568–576. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- 79.Iso H, Date C, Yamamoto A, Toyoshima H, Wantanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Am J Epidemiol. 2005;161:170–179. doi: 10.1093/aje/kwi027. [DOI] [PubMed] [Google Scholar]
- 80.Janzon E, Hedblad B, Berglund G, Engström G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255:266–272. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 81.Willemse BWM, Postma DS, Timens W, ten Hacken NHT. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23:464–476. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 82.Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Antó JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–1635. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- 83.Hjellvik V, Tverdal A, Furu K. Body mass index as predictor for asthma: a cohort study of 118,723 males and females. Eur Respir J. 2010;35:1235–1242. doi: 10.1183/09031936.00192408. [DOI] [PubMed] [Google Scholar]
- 84.Barret ST, Bevins RA. Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol Biochem Behav. 2013;114–115:9–15. doi: 10.1016/j.pbb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grimm JW, Ratliff C, North K, Barnes J, Collins S. Nicotine increases sucrose self-administration and seeking in rats. Addict Biol. 2012;17:623–633. doi: 10.1111/j.1369-1600.2012.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirshenbaum A, Green J, Fay M, Parks A, Phillips J, Stone J, Roy T. Reinforcer devaluation as a consequence of acute nicotine exposure and withdrawal. Psychopharmacology (Berl) 2014;232:1583–1594. doi: 10.1007/s00213-014-3792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palmatier MI, Lantz JE, O’Brien LC, Metz SP. Effects of nicotine on olfactogustatory incentives: preference, palatability, and operant choice tests. Nicotine Tob Res. 2013;15:1545–1554. doi: 10.1093/ntr/ntt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pavlos P, Vasilios N, Antonia A, Dimitrios K, Georgios K, Georgios A. Evaluation of young smokers and non-smokers with Electrogustometry and Contact Endoscopy. BMC Ear Nose Throat Disord. 2009;9:9–7. doi: 10.1186/1472-6815-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 90.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers & Prev. 2009;18:876–883. doi: 10.1158/1055-9965.EPI-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers & Prev. 2007;16:2479–2485. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 92.Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14:1270–1290. doi: 10.1093/ntr/nts159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3:a012112–a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- 95.McFadden KL, Cornier MA, Tregellas JR. The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol. 2014;5:553. doi: 10.3389/fpsyg.2014.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lea SE. The psychology and economics of demand. Psychol Bull. 1978;85:441–466. doi: 10.1037/0033-2909.85.3.441. [DOI] [Google Scholar]
- 98.West R, May S, McEwen A, McRobbie H, Hajek P, Vangeli E. A randomised trial of glucose tablets to aid smoking cessation. Psychopharmacology (Berl) 2009;207:631–635. doi: 10.1007/s00213-009-1692-3. [DOI] [PubMed] [Google Scholar]
- 99.Helmers KF, Young SN. The effect of sucrose on acute tobacco withdrawal in women. Psychopharmacology (Berl) 1998;139:217–221. doi: 10.1007/s002130050707. [DOI] [PubMed] [Google Scholar]
- 100.Lucas F, Sclafani A. Food deprivation increases the rat’s preference for a fatty flavor over a sweet taste. Chem Senses. 1996;21:169–179. doi: 10.1093/chemse/21.2.169. [DOI] [PubMed] [Google Scholar]
- 101.Sclafani A, Ackroff K. Deprivation alters rats’ flavor preferences for carbohydrates and fats. Physiol Behav. 1993;53:1091–1099. doi: 10.1016/0031-9384(93)90364-l. [DOI] [PubMed] [Google Scholar]
- 102.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tobacco Control. 2013;22:i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013;15:1003–1013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. 1998;7:281–293. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benowitz NL, Jacob P. Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- 106.Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring) 2007;15:1311–1318. doi: 10.1038/oby.2007.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


