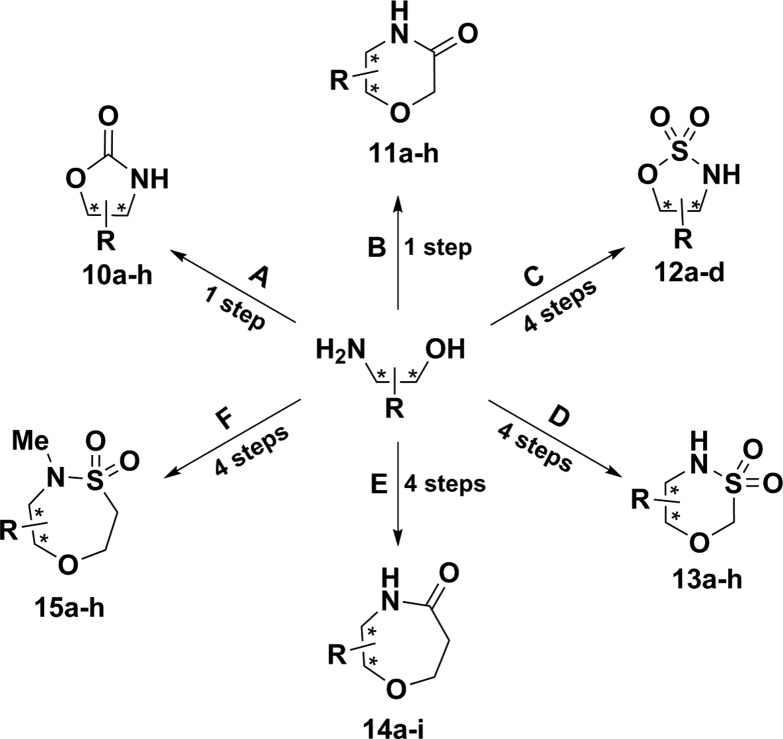
Scheme 1.
Reaction conditions: (A) CDI, Et3N, THF, 60 °C, 24 h, 27–85%; (B) NaH; ethyl chloroacetate, THF, rt, 24 h, 19–82%; (C) (i) Boc2O, Et3N, DCM, 0 °C, rt, 24 h, 83-99%; (ii) SOCl2, imidazole, Et3N, DCM, −40 °C, 2 h; then at rt for 2 h, 55–94%; (iii) RuCl3, NaIO4, MeCN:H2O (1:1), 0 °C to rt, 3 h, 31–95%; (iv) TFA, DCM, rt, 24 h, 66–90%; (D) (i) ClCH2SO2Cl, Et3N, DCM, 0 °C to rt, 6 h, 34-84%; (ii) PMB-Br, K2CO3, DMF, rt, 1 h, 17–89%; (iii) Cs2CO3, DMF, 80 °C, 24 h, 55–78%; (iv) CAN, MeCN:H2O (9:1), rt, 24 h, 24–78%; (E) (i) Boc2O, Et3N, DCM, 0 °C, rt, 24 h, 83–99%; (ii) t-butyl acrylate, Cs2CO3, t-BuOH, 24 h, 75–99%; (iii) 4.0 M HCl in dioxane, 24 h, 97–99%; (iv) T3P, Et3N, dioxane, 24 h, rt, 33–86%; (F) (i) TBS-Cl, Et3N, DCM, 0 °C to rt, 24 h, 71–95%; (ii) chloroethane sulfonyl chloride, Et3N, 0 °C to rt, 2 h, 75–95%; (iii) MeI, K2CO3, MeCN, 72 h, 70–90%; (iv) TBAF, THF, 2 h, 38–91%.