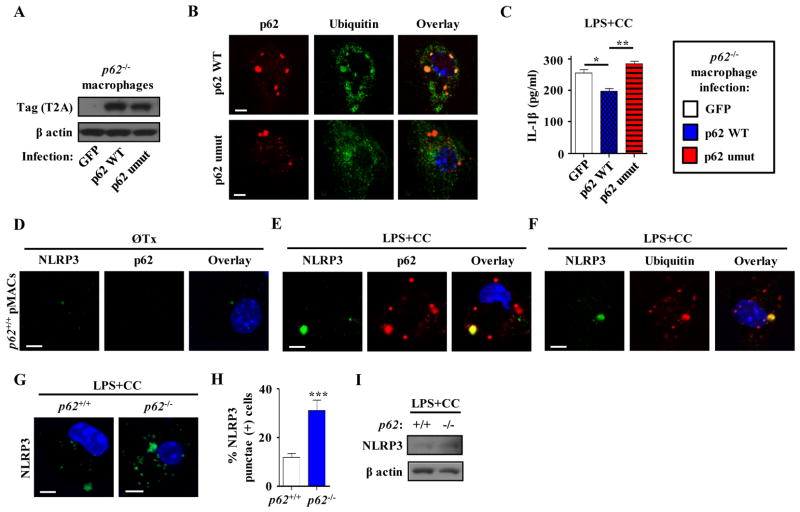
Figure 6. The p62 ubiquitin-binding domain is necessary to decrease secretion of IL-1β from p62−/− macrophages.
(a) Representative Western blot analysis of T2A tag after expression of constructs encoding control GFP, p62 WT or a form of p62 that cannot bind ubiquitin (umut) in p62−/− peritoneal macrophages in three independent experiments. (b) Immunofluorescence images of p62−/− peritoneal macrophages expressing either p62 WT or p62 umut constructs using antibodies against ubiquitinated proteins (FK-1) and p62. Images are representative of three independent experiments. (c) p62−/− peritoneal macrophages expressing either control GFP, p62 WT or p62 umut constructs were treated with LPS +/− CC. Cell culture media was assayed for IL-1β by ELISA (n=3 independent wells for each treatment). (d–f) Immunofluorescence images of peritoneal macrophages either without treatment (d) or after 12 hours LPS+CC treatment (e,f) using antibodies against NLRP3, p62, and ubiquitinated proteins (FK-1). Images are representative of three independent experiments. (g,h) Immunofluorescence images of control (p62+/+) and p62−/− peritoneal macrophages after LPS+CC treatment using an antibody against NLRP3 (g). Graph represents percent of cells containing NLRP3 punctae (n≥225 cells for each group; three independent experiments; h). (i) Western blot analysis of NLRP3 in LPS+CC treated cell lysates of control (p62+/+) and p62−/− peritoneal macrophages in three independent experiments. For all panels, data are presented as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Scale bar, 5 μm.