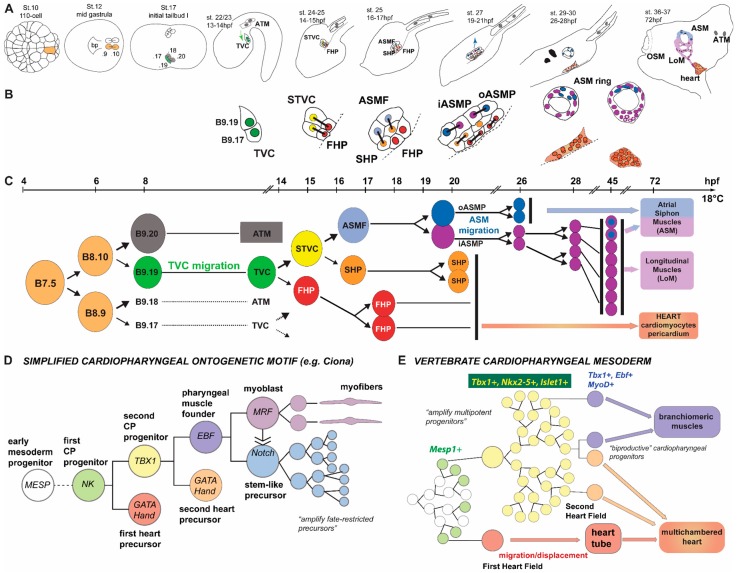
Figure 5.
Summary of cardiac cell lineage specification in Ciona. (A) Schematic embryos, larvae, and juvenile showing the B7.5-derived cardiopharyngeal lineage, with approximate hours post-fertilization (hpf) and stages according to [59]. Numbers in the left second and third panels are simplified version of cell names according to Conklin (1905) nomenclature and corresponding the (C). Arrows indicate collective TVC (green) and ASMP (blue) migrations. TVC: trunk ventral cells; ATM: anterior tail muscles; STVC: second TVC; FHP: first heart precursor; SHP: second heart precursor; ASMF: atrial siphon muscle founder cells; iASMP: inner atrial siphon muscle precursor; oASMP: outer atrial siphon muscle precursor; LoM: longitudinal body wall muscles (note that they derive from the iASMPs); OSM: oral siphon muscles, derived from the A7.6 lineage [60]; bp: blastopore; and (B) close-up views of cardiopharyngeal lineage cells. Black bars link sister cells (as per the lineage shown in C). White asterisks in the ASM ring at ~72 hours post fertilization indicate iASMP-derived LoM precursors that reactivate Mrf and the body wall muscle differentiation program [31]. Note that the relative contributions of the FHP and SHP to the different parts of the differentiated heart (epicardium, myoepithelium, raphe, and pacemakers) remain elusive; (C) Lineage representation of cadiopharyngeal mesoderm development. Color codes correspond to those used in A and B. Only the progeny of one TVC for one side of the animal is shown, but this pattern is reiterated 4 times in each developing embryo, such that the Ciona equivalent of the first and second heart fields (see B) are composed of distantly-related FHP and SHP. For example, the left second heart field is composed of the anterior/leader and posterior/trailer SHPs, whose last common ancestor is the Mesp+ left B7.5 blastomere; (D) simplified cardiopharyngeal ontogenetic motif as seen in Ciona, illustrating that in ascidian embryos, cell fates are first restricted to a few progenitors, which are secondarily amplified (as seen for both the ASMP, and heart precursors); and (E) schematic representation of equivalent cardiopharyngeal ontogeny in vertebrates, where multipotent progenitors from larger populations in the early embryos, these morphogenetic fields are patterned by cell-cell signaling causing spatially-defined progressive fate restrictions such that not every single multipotent progenitor expresses its full potential. This may explain why a small fraction (~10%) of the bipotent cardiopharyngeal progenitors produce both skeletal head muscles and SFH-derived cardiomyocytes, whereas these populations are easily switched to one fate or another experimentally. The existence of such plastic populations of multipotent progenitors probably fostered the diversification of cardiopharyngeal structures in vertebrates.