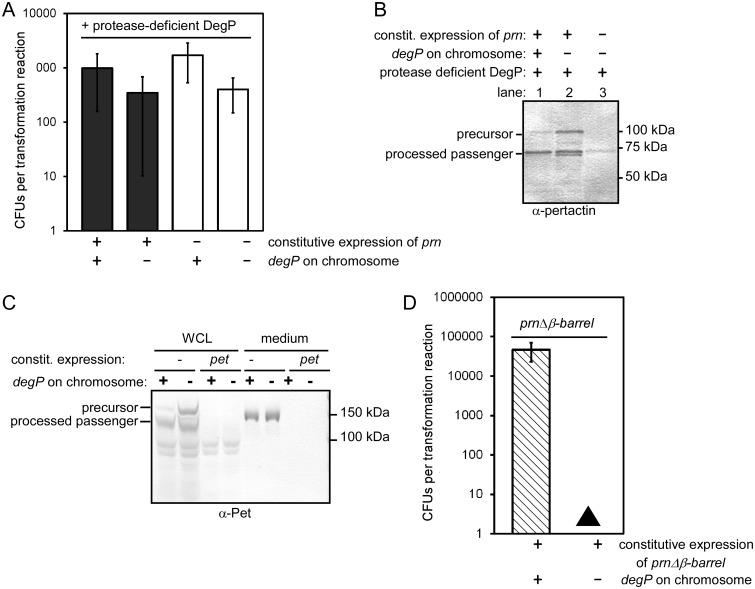
Fig 3. Differential requirement of the DegP protease and chaperone activity during expression of model ATs.
(A) E. coli viability as a function of degP, with or without constitutive expression of prn. To test if the DegP chaperone alone is sufficient to restore viability, a protease deficient version of DegP [16] was produced by co-transforming a plasmid expressing degP S210A. (B) Expression of prn results in production of the precursor and processing of the passenger (samples shown here were collected from cells described in (A). The role of the DegP protease for pertactin accumulation and secretion was assessed by expressing prn when only the DegP chaperone was present, versus the DegP chaperone plus the chromosomal wild type DegP. In the presence of the DegP protease activity, less pertactin precursor accumulates (compare lanes 1 vs. 2). (C) Wild type pet was expressed overnight in E. coli with or without a chromosomal copy of degP (same samples as grey bars in Fig 2A). In the absence of DegP, more full-length precursor accumulates in whole cell lysates (WCL). The yield of secreted Pet in the media after overnight production was not significantly altered with or without DegP. (D) After transforming prnΔβ-barrel in the E. coli degP null strain, very small colonies appeared after prolonged incubation (black triangle). (A, D) Error bars are standard deviations from at least three biological repetitions.